Abstract
Background. Beta emitters, such as 90Y, are increasingly being used for cancer treatment. However, beta emitters demand other precautions than gamma emitters during preparation and administration, especially concerning shielding. Aim. To discuss practical precautions for handling beta emitters in general, and specifically determine proper shielding for 90Y, while comparing to 177Lu and 131I. The aim is achieved through the application of physical principles combined with results from practical experience. Material and methods. Typical and maximal electron ranges were calculated for 131I, 177Lu, and 90Y, using data from a freely available database. Bremsstrahlung yields were calculated for 90Y shielded by lead, aluminium, or perspex. Bremsstrahlung spectrum from 90Y shielded by perspex was measured, and attenuation of spectrum by lead was calculated. Whole-body and finger doses to persons preparing 90Y-Zevalin were measured. Conclusions. Good laboratory practice is important to keep radiation doses low. To reduce bremsstrahlung, 90Y should not be shielded by lead but instead perspex (10 mm) or aluminium (5 mm). Bremsstrahlung radiation can be further reduced by adding a millimetre of lead on the outside of the primary shielding material. If suitable shielding is used and larger numbers of handlings are divided among several persons, then handling of beta emitters can be a safe procedure.
The use of radioactive nuclides for cancer therapy is increasing. This includes not only “old” isotopes such as iodine-131 (131I), but also “new” isotopes such as yttrium-90 (90Y) and lutetium-177 (177Lu). These isotopes emit short-ranged beta radiation (electrons), making them suitable for targeted radio-therapy. If the radioactive isotope is specifically taken up by the cancer cells, or can be attached to a pharmaceutical specifically taken up by the cancer cells, then it is possible to deliver a very high radiation dose to the cancer tissue while non-cancer tissue is spared.
In our department we recently started using 90Y-Zevalin® for the treatment of non-Hodgkins lymphoma. Being in a nuclear medicine department, our staff is experienced in handling gamma emitters, but we have less experience with beta emitters. The aim of this paper is to give a background for understanding the problems involved in handling and shielding beta emitters, present theoretical and practical results on the subject, and use these data to determine proper shielding. Examples of solutions used in our department will be given. The focus will be on 90Y, while theoretical results on 131I and 177Lu are presented for comparison.
Theory
90Y has a half-life of 2.7 days, and decays by emitting an electron (β− decay). The decay is to the ground state of the stable nucleus 90Zr, making 90Y a pure beta emitter with no accompanying gamma radiation.
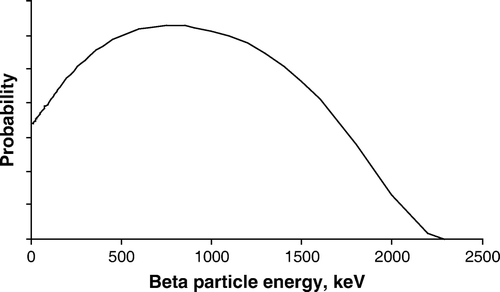
Along with the electron, an anti-neutrino is emitted. The anti-neutrino is a small particle with no electric charge and possibly no rest mass. It does not interact with the patient, other persons around, or our measuring apparatus. So we need not care about the anti-neutrino, except to note that it carries away some of the energy, meaning that the electron (beta particle) will have a spectrum of energies instead of a single, well-defined energy. In the case of 90Y the electron energy (beta spectrum) ranges from 0 to 2 284 keV, with a mean energy of 933 keV, see . These relatively high energies necessitate the use of well-considered shielding when handling the isotope. Once the isotope is injected into the patient, the radiation exposure to the surroundings is practically zero Citation[1].
Unlike x-rays and gamma radiation, electrons are completely stopped after a certain thickness of shielding material. However, in the process of being slowed down electrons may emit bremsstrahlung (“braking radiation”). This radiation consists of photons, and the process is utilized in x-ray tubes for the production of x-rays. When we are shielding against electrons we want to minimize the production of bremsstrahlung, as it constitutes a source of radiation exposure to the otherwise shielded surroundings. The intensity of bremsstrahlung is very dependent on the material hit by the electrons, with higher intensities emitted from materials with high atomic numbers than materials with low atomic numbers.
Material and methods
For the theoretical calculations, tabulated data of:
beta spectrum and mean beta energy for given isotope;
electron range for given energy in given material;
radiation yield of bremsstrahlung for given electron energy in given material;
attenuation coefficients for given photon energy in given element (lead)
were taken from the freely available Windows software Radiological Toolbox v. 2.0.0 Citation[2].
To give a rough measure of beta radiation range in tissue, the electron range in water (density 1.0 g/cm3) was calculated for maximal and mean beta energy for each of the isotopes 131I, 177Lu, and 90Y, using linear interpolation on the tabulated data. Three possible shielding materials were investigated: Lead, aluminium, and polymethyl methacralate (commonly known as perspex or plexiglass). To determine the thickness of shielding material necessary to stop all electrons emitted, the range of the most energetic electrons in the beta spectra was calculated for each combination of isotope and shielding material. Density of lead, aluminium and perspex were taken to be 11.34 g/cm3, 2.70 g/cm3, and 1.19 g/cm3, respectively. For 90Y, also the mean energy emitted as bremsstrahlung pr. beta particle incident on the shielding material was calculated for each shielding material. For this calculation, radiation yield was multiplied with probability density (beta spectrum), and the product was numerically integrated from 0 to highest electron energy.
Bremsstrahlung spectrum from a 90Y sample shielded by 10 mm perspex was measured with a Siemens e.soft gamma camera without collimator. The camera was equipped with a standard 3/8” NaI(Tl) crystal. To achieve larger effective crystal thickness the sample was not placed directly in front of the crystal but some meters to the side, such that the radiation reached the crystal with an incident angle of about 20°. Taking effective crystal thickness to be tripled, about 60% of 300 keV photons should be detected.
To determine the effect of lead shielding on the outside of perspex, the measured bremsstrahlung spectrum was multiplied by energy-dependent attenuation for 1 mm lead. Energy-absorption attenuation coefficients were used for the calculation, i.e., counting only locally absorbed energy as attenuated (broad beam attenuation). Since some of the scattered or re-emitted photons may later be absorbed, this gives a conservative estimate of the effectiveness of the shielding. The alternative of using total-absorption attenuation coefficients, i.e., counting scattered and re-emitted photons as attenuated (narrow beam attenuation), would have been too optimistic from a radiation protection point of view. These photons may have different directions than the original photon, but they still constitute a source of exposure.
All members of our staff are monitored with film dosimetry, worn at the chest. The films are processed on a monthly basis (tri-monthly for secretaries). The design of the film holder makes it possible to distinguish between dose from penetrating radiation (i.e., γ and x-rays) and non-penetrating radiation (i.e., β). The films are processed by the National Institute of Radiation Protection (Statens Insitut for Strålebeskyttelse, Denmark).
During two preparations of 90Y-Zevalin, finger doses were measured with ring dosimeters. The rings were worn at the base of left and right ring finger, measuring dose on the palm side. Shielding devices described in the Discussion section were used during the preparations. Each ring dosimeter consisted of a copper ring with a TLD tablet held in place by shrinking plastic. The ring dosimeters were prepared, calibrated, and processed by the National Institute of Radiation Protection. TLD material was LiF, calibrated against a Sr/Y-90 source, and precision was reported to be ±15% (1 SD). Courtesy of Svend Hvidsten, Odense University Hospital, results are also presented for fingertip measurements using equivalent TLD tablets and similar shielding. In each preparation, 1 500 MBq of 90Y was handled, and about 1 200 MBq of 90Y-Zevalin was prepared.
Results
Properties of 131I, 177Lu, and 90Y are compared in , along with ranges of beta particles in water and the three shielding materials considered. Calculated bremsstrahlung yields from shielded 90Y are presented in .
Table I. Comparison of three beta-emitters used for radiation therapy
Table II. Mean bremsstrahlung yield pr. 90Y decay when shielded by lead, aluminium, or perspex
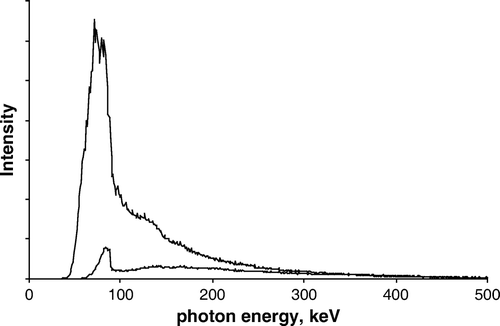
Measured bremsstrahlung spectrum from 90Y shielded by 10 mm perspex is shown in . In the same figure is also shown the attenuated spectrum, calculated for 1 mm lead.
Figure 2. Upper curve: Measured bremsstrahlung spectrum. Lower curve: Attenuated bremsstrahlung spectrum, calculated for 1 mm lead.
During an early test preparation of 90Y-Zevalin, the laboratory table was contaminated with drops of 90Y solution. Decontamination was made at once by one of the persons present, using no shielding. Afterwards, film dosimetry showed that the persons present at the incident had received skin doses (non-penetrating radiation) of 0.5, 0.7, 2.0, and 10 mSv, with the highest dose received by the person doing the decontamination. These doses were attributable to this single exposure. Corresponding one-month effective doses (whole-body doses) were 0.2, 0.1, 0.1, and 0.6 mSv, respectively.
In the following year 11 preparations and injections of 90Y-Zevalin were made by a team of 4 persons, using perspex shielding (cf. Discussion section). Care was taken to avoid contaminations. Film dosimetry showed that these persons had received one-year skin doses of 2.0, 2.4, 3.9, and 3.9 mSv.
Results on finger and fingertip doses are presented in .
Table III. Finger doses measured with TLD tablets for three individual preparations. In each case approximately 1 200 MBq of 90Y-Zevalin was prepared by one person working with 1 500 MBq 90Y.
Discussion
To keep radiation exposure as low as reasonably achievable (the ALARA principle) staffs handling radiopharmaceuticals must maintain good laboratory practice. This includes the use of tongs for handling of radioactive solutions, doing work effectively to keep exposure time low, and using proper shielding for reducing exposure rate. The principles of distance and time are the same whether the isotope handled is a gamma or a beta emitter, whereas optimal shielding depends on both the type and energy of the radiation. Three beta-emitting radioisotopes have been considered here.
The classical radioiodine, 131I, is a beta emitter, but the beta decay is immediately followed by a gamma decay of the excited daughter nucleus, meaning that one has to shield as much against gamma radiation as against beta radiation. The data in on 131I are presented here mainly for comparison.
177Lu is gaining use for cancer therapy. The short range of its beta radiation makes it suitable for treatment of micro-metastasis, as most of the radiation energy is distributed within a submillimetre range from the site of the decay. As seen in , the beta radiation needs only relatively thin shielding. 177Lu is not a pure beta emitter; the beta decay may be followed by emission of a photon of energy 208 keV (11%) or 113 keV (6%). From a clinical perspective this has the advantage that the distribution of the isotope can be visualized by a gamma camera. From a radiation protection perspective, the gamma radiation must be considered when shielding is chosen. Three millimetres of lead will attenuate 208 keV photons to 4% (of the 11%) and for practical purposes completely absorb 113 keV photons. As the beta energy is low, the problem of bremsstrahlung found with 90Y (see below) will be small for 177Lu.
90Y is increasingly being used for nuclear cancer therapy. The isotope emits beta radiation with high energy, suitable for treating tumours in the centimetre range. However, if care is not taken by the persons preparing and injecting the radiopharmaceutical, they may receive relatively high radiation doses, and there has been at least one report of a radiation accident among nuclear medicine staff handling 90Y Citation[3]. 90Y is a pure beta emitter with no accompanying gamma radiation, and as seen by , even the most energetic electrons emitted can be stopped by less than 2 mm lead. However, the energy re-emitted as bremsstrahlung (x-rays) during the slowing-down process is relatively high when lead is used as shielding material (cf. ). For this reason, shielding materials with low atomic numbers, such as aluminium or perspex, are better choices.
From a practical perspective, perspex has the advantage of being transparent. Be aware, however, that perspex can be damaged by cleaning with alcohol. This was realized the hard way by this author: One of our syringe shieldings developed large cracks shortly after being sprayed with alcohol by yours truly, maybe because of the temperature drop at the surface when the alcohol evaporated. We now disinfect perspex shielding with soap water.
Rounding the figures in , we find that 10 mm perspex or 5 mm aluminium is adequate shielding for 90Y. One should not be scared about the difference between 10 and 10.3 mm or 5 and 5.2 mm, since even the very seldom electron emitted with the highest possible energy (which might pass the full 10 mm of perspex or 5 mm of aluminium) will have lost virtually all its energy on the way, thus having almost no energy left for delivering radiation dose beyond the shielding.
However, even when a low-atomic-number shielding material is used, some bremstrahlung will be emitted. Recorded bremsstrahlung spectrum after 10 mm perspex is shown in . As a gamma camera is optimized for detection of photons in the range approximately 100 – 200 keV, the recorded spectrum needs a few comments. At the lowest energies the spectrum unrealistically falls to zero. This is because the crystal in the camera is shielded from low energy radiation to avoid noise in clinical imaging. At high energy the recorded spectrum will underestimate the true spectrum, because high-energy photons may pass the crystal undetected, although this has been anticipated by the recording setup (cf. Material and methods section). While the spectrum shown is not the full truth, it is useful to give a rough measure of the bremsstrahlung spectrum from ∼70 keV to ∼300 keV, and an idea of the spectrum above this range. Note specifically that some of the bremsstrahlung is emitted with high energies, in principle up to the maximal electron energy.
Also seen in is the impact of adding 1 mm lead to the outside of the primary perspex shielding. As can be seen, the lead has a profound impact on bremsstrahlung with energies up to about 200 keV, whereas it has little effect at energies above 300 keV. Since most of the bremsstrahlung is emitted in the lower energy range, the overall effect is a considerable reduction of radiation beyond the shielding.
Vial and syringe shielding for 90Y used in our department are shown in . For the vial in use during the longest time of preparation we use a container of perspex surrounded by lead glass (left). This container provides very good shielding, but is rather bulky. For shorter-period aspects of the preparation, a container of 10 mm perspex without secondary shielding is used (middle). Syringes are shielded with 10 mm perspex (right).
Figure 3. Example of shielding. Left to right: Vial shielding made of perspex surrounded by lead glass, mini-vial shielding made of perspex, and syringe shielding made of perspex.

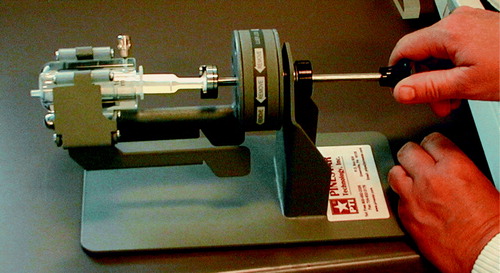
Finally, staffs must also be aware of radiation exposure during injection. Once 90Y is fully injected in the patient, the patient's body gives very good shielding for his or her surroundings. But during the injection especially the fingers of the injecting person may be exposed. This problem is enlarged by the fact that therapy doses may have to be given slowly; e.g., 90Y-Zevalin must be injected over 10 minutes. In our department we use the manual injector shown in . The injector increases distance from fingers to activity, while at the same time providing shielding in the backward direction not covered by the perspex shielding.
From the measured doses reported here we see that care must be taken in the handling of 90Y. Relatively large radiation doses (for laboratory work) may occur, as illustrated by the results of the contamination incident, giving 10 mSv skin dose and an unknown finger dose. Good effect of proper shielding is seen by the finger and body doses reported from clinical situations. For comparison, The International Commission on Radiological Protection (ICRP) recommends annual dose limits of 500 mSv for extremities/hands and for the skin (150 mSv for the lens of the eye) Citation[4], Citation[5]. No measured finger or fingertip dose was above 7 mSv. Adding a factor of two for safety, we find a limit of 35 preparations pr. person pr. year. These numbers refer to the handling of 1 500 MBq 90Y during preparation of typically 1 200 MBq 90Y-Zevalin.
For further reading on radiation protection in 90Y-therapies, the reader is recommended the paper by Cremonesi et al. Citation[3]
Conclusion
Beta emitters present other shielding issues than gamma emitters. Once the beta emitter is inside the patient, almost no radiation is given off to surroundings, but good laboratory practice (GLP) and suitable shielding are mandatory during preparation and injection of the radioactive isotope. For high-energy beta emitters like 90Y, lead is less suitable as primary shielding material because of bremsstrahlung. 10 mm perspex or 5 mm aluminium provides good shielding of beta radiation from 90Y without much bremsstrahlung production. Adding a millimetre of lead on the outside of the primary shielding further reduces bremsstrahlung. With GLP and suitable shielding, the handling of 90Y can be a safe procedure, but if the number of preparations during a year is large, they should be divided among several persons.

Acknowledgements
Additional data were presented from Department of Nuclear Medicine, Odense University Hospital, courtesy of Svend Hvidsten. The National Institute of Radiation Protection very helpfully provided and processed dosimeters for finger measurements.
References
- Zanzonico PB, Binkert BL, Goldsmith SJ. Bremsstrahlung radiation exposure from pure beta-ray emitters. J Nucl Med 1999; 40: 1024–8
- Eckerman KF, Sjoreen AL. Radiological Toolbox. Oak Ridge National Laboratory 2006. Available at: http://www.nrc.gov/about-nrc/regulatory/research/radiological-toolbox.html [ accessed 15-5-2008]
- Cremonesi M, Ferrari M, Paganelli G, Rossi A, Chinol M, Bartolomei M, et al. Radiation protection in radionuclide therapies with 90Y-conjugates: risks and safety. Eur J Nucl Med Mol Imaging 2006; 33: 1321–7
- ICRP 60. 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP 1991;21:1–201.
- ICRP 103. The 2007 Recommendations of the International Commission on Radiological Protection. Ann ICRP 2007;37:1–332.