Abstract
Introduction. Antiprotons have been proposed as a potential modality for radiotherapy because the annihilation at the end of range leads to roughly a doubling of physical dose in the Bragg peak region. So far it has been anticipated that the radiobiology of antiproton beams is similar to that of protons in the entry region of the beam, but very different in the annihilation region, due to the expected high-LET components resulting from the annihilation. On closer inspection we find that calculations of dose averaged LET in the entry region may suggest that the RBE of antiprotons in the plateau region could significantly differ from unity, which seems to warrant closer inspection of the radiobiology in this region. Materials and Methods. Monte Carlo simulations using FLUKA were performed for calculating the entire particle spectrum of a beam of 126 MeV antiprotons hitting a water phantom. Results and Discussion. In the plateau region of the simulated antiproton beam we observe a dose-averaged unrestricted LET of about 4 keV/µm, which is very different from the expected 0.6 keV/µm of an equivalent primary proton beam. Even though the fluence of secondaries is a magnitude less than the fluence of primary particles, the increased stopping power of the secondary particles causes an increase in the dose averaged LET which is expected to result in a RBE different from unity.
Antiprotons as a new beam modality in radiotherapy are being investigated by the AD-4/ACE collaboration since 2003. A beam of antiprotons hitting a water phantom exhibits a similar depth-dose curve as that known from protons, except that the Bragg peak is significantly more pronounced due the annihilation events occurring at the end of the antiproton particle tracks.
Holzscheiter et al. Citation[1–3] investigated the radiobiology of antiprotons using an antiproton beam with kinetic energy of 50 MeV from the AD facility at CERN.
Since the dosimetry of the antiproton beam at CERN is a non-trivial matter for several reasons, the relative biological effect (RBE) in the peak region could not be measured at the time. Instead the AD-4 collaboration concentrated on measuring the ratio of the biological effect between the peak and plateau area (defined as “Biological Effective Dose Ratio”, or, “BEDR”), which is measurable irrespectively of the deposited dose Citation[3]. The BEDR value therefore expresses quantitatively, how much one can reduce the dose in the plateau for a constant effect in the peak.
In this paper we calculate the linear energy transfer (LET) spectrum and the dose averaged LET for a beam of 126 MeV antiprotons hitting a water target. This energy was chosen as it matches the beam energy in our current radiobiological experiments. All references to LET are meant to be unrestricted LET, i.e. LETinf.
Since antiprotons have the same stopping power as protons in the clinical relevant energy interval, it has so far been assumed that antiprotons exhibit the same radiobiology as protons in the entrance channel. The contribution from secondary particles arising from in-flight annihilation of the primary beam was considered insignificant. Using Monte Carlo calculations for the dose of the primary beam in the peak, it is then possible to provide an estimate of the RBE in the peak region by assuming RBE = 1 in the plateau, e.g. in Citation[3] the best estimate of the peak RBE is 2.25 for 20% clonogenic survival of V79 Chinese hamster cells.
More recently, antiproton dose calculations with FLUKA Citation[4], Citation[5] were successfully benchmarked against experimental measurements with ionization chambers Citation[6]. Furthermore, particle spectra calculated with FLUKA were used in conjunction with response models in order to calculate the response of alanine detectors exposed to the mixed radiation field Citation[7]. This allowed deducing a measurement for the dose deposit along the entire depth-dose curve. Measurements of RBE are therefore now possible and were performed in 2007. These findings are to be published in a future article.
Methods
FLUKA version 2006.3 is used for our calculations performed for this paper. A 5×5 cm2 square field of 502 MeV/c (∼126 MeV) antiprotons with a momentum spread of Δp/p = 0.5% and 5 mrad divergence is dumped into a water phantom. The range in water for this beam is approximately 11.5 cm. The scoring along the beam in the water target is done in rectangular boxes covering an area of 2×2 cm2 laterally and a thickness of 1 cm along the beam line. The scoring region is thus significantly smaller than the beam width, in order to achieve lateral equilibrium of particles, which scatter into and out of the scoring volume. The other beam parameters were chosen in order to mimic the beam which we have available at CERN. Custom FLUKA user routines were written in order to extract the fluence for each particle species as a function of energy per nucleon. The stopping power used for the LET averaged calculations is provided by PSTAR, ASTAR and MSTAR routines developed by Berger et al. Citation[8] and Paul et al. Citation[9].
The track averaged and the dose averaged LET is calculated according to Equations 1 and 2, respectively:1
2 where (Ej,Zi) is the electronic stopping power in liquid water for each particle with charge Zi at energy Ej, measured at the center of the respective energy bin, φ[Ej,Zi] and D[Ej,Zi] are the track length fluence and dose of the corresponding particle, respectively. The sum is taken over all particle charges up to Zproj=6 and over the entire energy spectrum obtained from FLUKA up to 1 GeV. Being presented a highly mixed radiation field with a strong content of high LET secondary particles we choose to use the dose averaged LET for this analysis.
Results
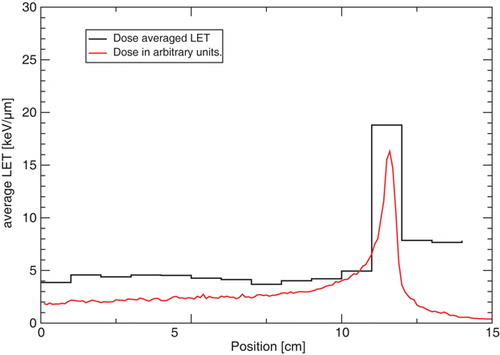
The complete LET-spectra for those two positions are shown in . The dose averaged LET is shown in . A calculated depth-dose curve for antiprotons in water is added in these figures in order to guide the reader. The plateau and peak averaged values are also presented in .
Figure 1. FLUKA calculation of charged particle LET spectrum of a 126 MeV antiproton beam as shown in Citation[6]. The spectrum was calculated both in the peak region and in the plateau region. Charged particles with 1 ≤ Z≤6 were taken into account. The sharp line in the plateau region at 0.6 keV/µm originates from the primary antiproton beam.
![Figure 1. FLUKA calculation of charged particle LET spectrum of a 126 MeV antiproton beam as shown in Citation[6]. The spectrum was calculated both in the peak region and in the plateau region. Charged particles with 1 ≤ Z≤6 were taken into account. The sharp line in the plateau region at 0.6 keV/µm originates from the primary antiproton beam.](/cms/asset/16f2545b-5678-4be8-8d83-59f87ed1616e/ionc_a_326840_f0001_b.jpg)
Table I. Calculated averaged LET.
Antiprotons with a kinetic energy of 126 MeV have a stopping power of 0.615 keV/µm in water. In the primary antiproton beam is clearly visible as the stopping power bin with the highest relative fluence. Even though the track averaged LET can be shown to be comparable to the stopping power of the primary beam, the dose averaged stopping power is a magnitude higher, due to the significantly higher LET of the low energy fragments present in the radiation field.
Discussion
The RBE dependence on LET has been discussed in several publications, often showing a peaking of the RBE at around 100 keV/µm. This LET corresponds to the mean distance between ionization events being comparable with the width of DNA strands, which is believed to effectively induce double strand breaks Citation[10]. Naturally, this is rather an oversimplification of the complex biological processes, e.g. if one takes a look at Figure 11 found in the ICRU Report # 16 Citation[11], which relates the RBE with LET, the clear dependence is less convincing. Furthermore RBE dependence cannot be single-valued, since several particles with different energies can have the same LET. However, for the discussion presented here it will be sufficient to conclude that RBE changes with LET.
Considering the work by Wouters et al. Citation[12], where the RBE of proton beams with V79 Chinese hamster cells was investigated, one may actually get the impression that the change in dose averaged LET presented in this paper may be sufficient to significantly alter the RBE. The distribution of secondary particles from annihilation, as calculated with FLUKA, shows that mainly fragments such as pions, protons and some Helium ions contribute to the dose. Since most of these fragments have a charge Z = 1 we here assume that the resulting RBE may possibly be very similar to an LET equivalent field consisting of only protons with various energies. Wouters directly measured the RBE as a function of depth in a spread-out Bragg peak (SOBP) of 21 mm width and a maximum energy of 70 MeV, corresponding to a maximum depth in water of 28 mm. For three different endpoints of clonogenic survival of 3, 50, and 80% he reports a range of RBE values between 1.2 and 1.6. He also calculates the dose-averaged linear energy transfer across the width of the SOBP using the weighted sum of range-shifted pristine Bragg peaks to span the interval from 2.5 to 6.0 kev/µm Citation[12].
Applying the dose averaged stopping power in the plateau presented here in conjunction with Figure 10 in reference Citation[12], we find an RBE in the plateau in the region of 1.2–1.3 for 10 to 50% survival, rather than 1. This could have significant consequences for the RBE estimate in the peak area described earlier by Holzscheiter et al. Citation[3]. For instance a RBE of 2.25 may increase to 2.7–3.0.
RBE measurements of an antiproton beam of 126 MeV energy were carried out in October 2007 and preliminary estimates sustain the findings presented here, although the Co-60 reference irradiations needed to extract a final RBE from these measurements are still to be performed.
Conclusion
Using FLUKA we have calculated the unrestricted LET spectrum of several ions. The maximum dose averaged LET in the Bragg peak region was estimated as 19 keV/µm, which suggests, that the RBE of antiprotons for V79 Chinese hamster cells may differ from unity. This would have a significant impact on earlier estimates for the RBE in the peak of an antiproton beam of 50 MeV stopping in a target of V-79 Chinese hamster cells embedded in gelatin and clearly should be considered in future analyses.
Acknowledgements
The Danish cancer society supported this project with a grant.
References
- Maggiore C, Agazarayan N, Bassler N, Blackmore E, Beyer G, DeMarco JJ, et al. Biological effectiveness of antiproton annihilation. NIM B 2004; 214: 181–5
- Holzscheiter MH, Agazarayan N, Bassler N, Beyer G, DeMarco JJ, Doser M, et al. Biological effectiveness of antiproton annihilation. NIM B 2004; 221: 210–4
- Holzscheiter MH, Bassler N, Agazaryan N, Beyer G, Blackmore E, DeMarco JJ, et al. The biological effectiveness of antiproton irradiation. Radiother Oncol 2006; 81: 233–42
- Fassò A, Ferrari A, Ranft J, Sala PR. FLUKA: A multi-particle transport code. CERN-2005-10, INFN/TC 05/11, SLAC-R-773.
- Fassò A, Ferrari A, Roesler S, Sala PR, Battistoni G, Cerutti F, et al The physics models of FLUKA: Status and recent developments. In: Computing in High Energy and Nuclear Physics 2003 Conference (CHEP2003), La Jolla, CA, USA, March 24-28 2003. (paper MOMT005), eConf C0303241 (2003), arXiv:hep-ph/0306267.
- Bassler N, Holzscheiter MH, Jäkel O, Kovacevic S, Knudsen HV, and the AD-4/ACE Collaboration The antiproton depth-dose curve in water. Phys Med Biol 2008;53:793–805.
- Bassler N, Hansen JW, Palmans H, Holzscheiter MH, Kovacevic S, and the AD-4/ACE Collaboration The antiproton depth dose curve measured with alanine detectors. NIM B 2008;266:929–36.
- Berger MJ, Coursey JS, Zucker MA, Chang J. Stopping-power and range tables for electrons, protons, and helium ions. http://physics.nist.gov/PhysRefData/Star/Text/contents.html.
- Paul H, Schinner A. MSTAR. http://www.exphys.uni-linz.ac.at/stopping/.
- Hall EJ. Radiobiology for the Radiologist5th ed. Lippincott Williams & Wilkins. 2000
- International Commission on Radiation Units and Measurements Linear energy transfer. Technical Report 16, ICRU, 1970.
- Wouters BG, Lam GKY, Oelfke U, Gardey K, Durand RE, Skarsgard LD. Measurements of relative biological effectiveness of the 70 MeV proton beam at TRIUMF using Chinese hamster V79 cells and the high-precision cell sorter assay. Radiat Res 1996; 146: 159–70