Abstract
Background. Sunitinib is a multitargeted tyrosine kinase inhibitor used in the treatment of metastatic renal cell carcinoma (RCC) and gastrointestinal stromal tumor (GIST), and undergoing evaluation for other malignancy. Hypertension is one of its major side effects with a substantial variation in the reported incidences among clinical studies. We here performed a systematic review and meta-analysis of published clinical trials to determine its overall risk. Methods. Relevant studies were searched and identified in MEDLINE (OVID 1966 to July, 2007), Web of Science, and abstracts presented at the American Society of Clinical Oncology annual meetings from 2004 through 2007. Eligible studies were prospective clinical trials that had described events of hypertension for patients who received single agent sunitinib. The incidence of hypertension and relative risk (RR) were calculated using the random-effects or the fixed-effects model. Results. A total of 4, 999 patients with RCC and other malignancies from 13 clinical trials were included for analysis. Among patients receiving sunitinib, the incidence of all-grade and high-grade hypertensions were 21.6% (95% CI: 18.7–24.8%) and 6.8% (95% CI: 5.3–8.8%) respectively. The risk may vary with tumor type and the dosing schedule of sunitinib. Sunitinib was associated with a significantly increased risk of high-grade hypertension (RR=22.72, 95% CI: 4.48 to 115.29, p<0.001) and renal dysfunction (RR: 1.36, 95% CI: 1.20 to 1.54, p<0.001) in comparison with controls. Conclusions. There is a significant risk of developing hypertension and renal dysfunction among patients receiving sunitinib. Adequate monitoring and treatment of hypertension is recommended.
Sunitinib malate (Sutent, SU11248, Pfizer) is a new orally administered anti-neoplastic agent with a broad-spectrum anti-tumor activity on cancer cell proliferation and angiogenesis in preclinical models Citation[1–3]. It is a highly potent inhibitor of multiple receptor tyrosine kinases including vascular endothelial growth factor receptors 1, 2, and 3 (VEGFRs), platelet-derived growth factor receptors α and β (PDGFP-α and PDGFR-β) FMS (Feline McDonough Sarcoma)-like tyrosine kinase 3 (Flt-3), c-Kit, and RET receptor tyrosine kinase Citation[1–5]. Significant clinical benefit of sunitinib has been demonstrated in patients with metastatic RCC in phase II and phase III clinical trials as the first or second line treatment Citation[6–8]. In addition, sunitinib is effective in the treatment of advanced GIST after failure of imatinib Citation[9]. The efficacy of sunitinib in other malignancies is currently undergoing extensive evaluation.
Although sunitinib is well-tolerated in many patients, significant toxicities are associated with its use. Fatigue, diarrhea and nausea are the most common adverse events experienced by patients, with neutropenia, elevation of lipase, and anemia being the most common laboratory abnormalities Citation[6–9]. Hypertension is a common side effect observed in these trials Citation[6–9]. Monitoring and management of hypertension is important because poorly controlled hypertension may lead to serious cardiovascular events and dose reduction. Indeed, declined cardiac function is more common in patients receiving sunitinib than that in controls Citation[6]. The left ventricular dysfunction might be exacerbated or caused in part by hypertension, in addition to a possible direct cardiomyocyte toxicity from sunitinib Citation[10]. Besides, cases of the more serious reversible posterior leukoencephalopathy syndrome related to hypertension were reported in patients receiving sunitinib Citation[11]. Because of the limited number of patients in each clinical trial, significant variation exits regarding the reported incidences of hypertension among these studies, ranging from 15.3 to 29.6% Citation[6–9]. Also, risk factors for the sunitinib-induced hypertension are unknown. In order to gain a better understanding of the overall risk of hypertension in cancer patients treated with sunitinib, we have conducted a systematic review of the literature to identify published clinical trials using sunitinib as a single agent, and performed a meta-analysis to determine the risk. We have also explored potential risk factors including tumor type and sunitinib dosing. In addition, we have investigated the risk of renal dysfunction associated with sunitinib to understand the underlying mechanism of hypertension.
Methods
Data source
We conducted an independent review of citations from MEDLINE database (OVID 1966-July 2007). The search included key words “sunitinib”, “SU11248”, “sutent”, and “cancer”, and was limited to English language and human studies. The search strategy also employed text terms such as “hypertension”. In addition, we manually searched all the abstracts that contain “sunitinib” presented at recent 2004–2007 American Society of Clinical Oncology (ASCO) annual meetings. We reviewed each of the publications, and included phase II and III clinical trials using sunitinib as a single agent either at a continuous daily dosing (37.5 mg daily) or intermittent dosing (50 mg daily for 4 weeks, followed by 2 weeks off, for a 6-week cycle). An independent search using the Web of Science database (a product developed by the Institute for Scientific Information, a citation database) was also conducted to ensure that there were no additional studies. We extracted details on study characteristics, patient characteristics, treatment information, results, and follow-up from these selected trials.
Study Selection
Sunitinib has been approved for the treatment of patients with advanced RCC and GIST as a single agent at 50 mg daily for 4 weeks, followed by 2 weeks off on a 6-week cycle (intermittent dosing). Thus it has practical significance to determine the risk of hypertension at this dosing level. Clinical trials are currently ongoing to evaluate the efficacy of continuous dosing at 37.5 mg daily (continuous dosing) for RCC and other malignancies. The scheduled total dose of sunitinib is the same between these two dosing schedules. The goal of this study is to determine the risk of hypertension at this scheduled total dose. Thus, any prospective clinical trials using sunitinib at this dose level with available data of hypertension were included for analysis. Phase I trials were excluded from analysis due to their various dose levels. Trials that met the following criteria were included: 1) prospective phase II, III, and expanded access clinical trials in cancer patients; 2) assignment of participants to the treatment with sunitinib as a single agent at 37.5 mg daily (continuous daily dosing) or 50 mg daily for 4 weeks, followed by 2 weeks off, for a 6-week cycle (intermittent dosing); and 3) events or event rate and sample size available for hypertension or creatinine increase.
Clinical end points
Hypertension and creatinine increase were extracted from the safety profile in each trial. These clinical end points were recorded according to versions II or III of the Common Terminology Criteria for Adverse Events (CTCAE) of National Cancer Institute (http://ctep.cancer.gov/reporting/ctc_archive.html) Citation[12]. Both versions describe the grading of hypertension as: grade I, asymptomatic, transient (<24 hrs) increase of blood pressure by >20 mmHg (diastolic) or to >150/100 mmHg if previously within normal limit (WNL), intervention not indicated; grade II, recurrent or persistent (>24 hrs) or symptomatic increase by >20 mmHg (diastolic) or to >150/100 mmHg if previously WNL, monotherapy may be indicated; grade III, requiring more than one drug or more intensive therapy than previously; and grade IV, hypertensive crisis. The grading for creatinine increase is divided into the following: grade I, between the upper limit of normal (ULN) and 1.5×ULN; grade II, 1.5-3.0×ULN; grade III, 3.0-6.0×ULN; grade IV, 6.0×ULN.
Statistical analysis
All statistical analysis was performed using the Comprehensive MetaAnalysis program (Version 2, Biostat, Englewood, NJ). The summary incidence of hypertension was calculated from patients receiving sunitinib in single-arm and randomized controlled clinical trials. For the calculation of relative risk, data was extracted only from randomized controlled studies, and the incidence of HTN in patients assigned to sunitinib was compared only with those assigned to control treatment in the same trial. A p-value of less than 0.05 was considered statistically significant.
For each meta-analysis, both fixed-effects model (weighted with inverse variance) and random-effects model were considered Citation[13]. The Cochran's Q statistic was first calculated to assess the heterogeneity among those trials included for analysis. For p-value less than 0.1, the assumption of homogeneity was deemed invalid Citation[14], and the random-effects model was used after exploring the underlying causes for the heterogeneity for possible exclusion of certain trials. Results from both the fixed-effects model and the random-effects model were evaluated. If the results were similar between the random-effects and fixed-effects models, only the fixed-effects model results were reported. A two-tailed p-value of less than 0.05 was judged as statistically significant.
Results
Search results
Our search yielded a total of 156 articles on sunitinib from the literature. After reviewing each publication, we identified 4 original studies fulfilled our inclusion criteria Citation[6–9]. From the abstracts published during recent ASCO annual meetings (2004–2007), we identified 51 abstracts that were related to sunitinib. After reviewing each abstract, we included 9 additional trials to our meta-analysis. A total of 13 clinical trials were included for this analysis (as shown in ), encompassing two randomized-controlled studies Citation[6], Citation[9], two expanded-access trials Citation[15], Citation[16], and nine single-arm phase II studies Citation[7], Citation[8], Citation[15–23]. Five trials were sponsored by Pfizer Inc Citation[6–8], Citation[15], Citation[16]. One trial was funded in part by Pfizer and by a Veterans Administration Merit Review Grant Citation[9]. The support for the other trials was not described.
Table I. Characteristics of clinical trials included in the meta-analysis.
Patients
A total of 4, 999 patients from 13 clinical trials were included for the meta-analysis. Baseline characteristics including sample size for the calculation of hypertension incidence are listed for each trial in . ECOG performance status for the majority of patients was between 0–1. Hypertension was not described as a pre-existing condition in any of the trials. Baseline renal function was described as “adequate”. Underlying metastatic malignancies include RCC, GIST, non-small cell lung cancer, gastric cancer, and urothelial carcinoma. Prior treatment included imatinib for GIST in one trial and bevacizumab for RCC in another trial. Majority of patients with RCC received nephrectomy Citation[6–8], Citation[15], Citation[21], Citation[23]. For the two randomized controlled studies, treatment options were randomly assigned, with one using a placebo Citation[9], and the other using interferon as a control Citation[6].
Incidence of hypertension
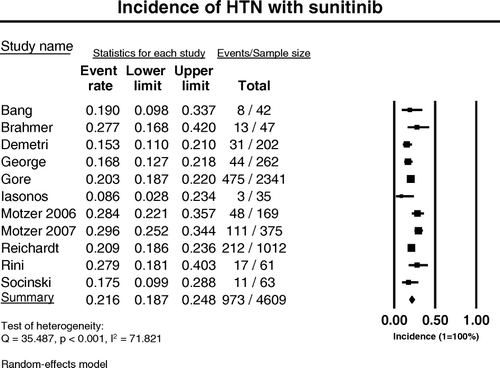
A total of 4, 609 patients with RCC, GIST, and other malignancy who received sunitinib treatment were included for this analysis. Majority of these patients have either RCC or GIST. The starting dose of sunitinib was 37.5 mg every day (continuous daily dosing) or 50 mg once a day for 4 weeks then 2 weeks off, on a 6-week cycle (intermittent dosing). Among patients receiving sunitinib with available data for analysis, the incidence of all-grade hypertension ranged between 8.6% and 29.6%, with the highest incidence seen in patients with metastatic renal cell carcinoma in the phase III randomized controlled trial by Motzer et al.Citation[6], and the lowest incidence observed in patients with urothelial carcinoma Citation[20]. Significant heterogeneity existed among these studies (Q = 35.49, p < 0.001) (), and all the studies were included for final analysis using the random-effects model. The calculated summary incidence of all-grade hypertension among patients receiving sunitinib from all these trials was 21.6% (95% CI: 18.7–24.8%) ().
Figure 1. Annotated forest plot for meta-analysis of the incidence of hypertension in cancer patients who received sunitinib. The summary incidence of all-grade hypertension was calculated using the random-effects model. The incidences and 95% confidence intervals for each study and the final combined result are displayed numerically on the left and graphically as a forest plot on the right. Under study name, the first author's name was used to represent each trial. If the same first author was involved in two trials, then the publication year was also included for identification of the trial. The size of the squares is directly proportional to the amount of information available. For individual trials: filled-in square, incidence; lines, 95% confidence interval; diamond plot, overall results of the included trials.
Incidence of high-grade hypertension
High-grade (grade 3 or 4) hypertension is associated with significant morbidity, and may result in dose reduction or discontinuation of sunitinib. The incidence of high-grade hypertension ranged between 2.4 and 14.8%, with the highest incidence seen in the phase II trial by Rini et al. in patients with bevacizumab-refractory RCC Citation[21], and the lowest incidence observed in patients with gastric cancer Citation[17]. The calculated summary incidence of high-grade hypertension among 4407 patients was 6.8% (95% CI: 5.3–8.8%) using the random-effects model (Test of heterogeneity: Q = 24.383, p = 0.004, I2=63.09), as shown in .
Table II. Incidence and relative risk of hypertension with sunitinib.
Incidence of hypertension in patients with RCC versus non-RCC malignancy
In order to explore the relationship between sunitinib-associated hypertension and tumor type, we have further analyzed the incidence of hypertension in patients with RCC and non-RCC cancers. Among patients with RCC, the summary incidences of all-grade and high-grade hypertension were 25.9% (95% CI: 20.0–32.8%, sample size: 2946) and 8.3% (95% CI: 5.6–12.1%, sample size: 3053) respectively using the random-effects model; while for those patients with non-RCC malignancies, the summary incidences of all-grade and high-grade hypertension were 19.6% (95% CI: 17.7–21.6%, sample size: 1663) and 5.3% (95% CI: 4.2–6.6%, sample size: 1354) respectively using the fixed-effects model.
Interestingly, there was a significant difference detected between RCC and non-RCC cancer in terms of the incidence of sunitinib-associated all-grade hypertension (RR 1.32, 95% CI: 1.18–1.48%, p < 0.001) and high-grade hypertension (RR 1.57, 95% CI: 1.22–2.02%, p = 0.001).
Incidence of hypertension in patients receiving continuous daily dosing versus intermittent dosing of sunitinib
In order to explore the relationship between the risk of hypertension and sunitinib dosing schedule, we have further analyzed the incidence of hypertension in patients receiving intermittent dosing of sunitinib (50 mg once a day for 4 weeks then 2 weeks off, on a 6-week cycle) versus continuous daily dosing (37.5 mg every day). Data were available for analysis in patients with non-RCC cancers. Among a total of 1556 patients receiving intermittent dosing of sunitinib, the summary incidence of hypertension was 16.9% (95% CI: 13.6–20.9%) using the random-effects model (Test of heterogeneity: Q = 10.059, p = 0.074, I2=50.296); among a total of 107 patients receiving continuous daily dosing of sunitinib, the summary incidence of hypertension was 27.1% (95% CI: 19.5–36.3%) based on the fixed-effects model (Test of heterogeneity: Q = 0.013, p = 0.909, I2=0.001). There was significant difference detected between continuous and intermittent dosing of sunitinib in terms of the incidence of hypertension (RR 1.60, 95% CI: 1.15–2.23%, p = 0.005).
Relative risk of hypertension
Relative risk (RR) calculated from a randomized-controlled trial can be used to determine specifically the contribution of sunitinib to the development of hypertension in these patients with many confounding factors such as underlying malignancy, renal function, and other therapeutic interventions. A meta-analysis of relative risk (RR) associated with sunitinib in comparison with controls was performed for the two randomized controlled trials in patients with GIST and RCC Citation[6], Citation[9]. One trial used placebo as a control, and the other trial used interferon as a control. The incidence of all-grade hypertension was low among the patients with RCC receiving interferon (3.6%, 95% CI: 2.1–6.1%), and was higher among patients with GIST receiving placebo (10.8%, 95%CI: 6.1–18.4%). The incidences of high-grade hypertension were minimal among the patients with RCC receiving interferon (1/360), and the patients with GIST receiving placebo (0/102). There was significant variation between patients with GIST and RCC in the relative risk of hypertension with sunitinib. For patients with GIST, the relative risk of hypertension with sunitinib is not statistically significant, with an RR of 1.42 (95% CI: 0.75 to 2.71, p = 0.28), while for patients with RCC, sunitinib is associated with a significant risk of hypertension with an RR of 8.20 (95% CI: 4.70 to 14.29, p < 0.001). Overall, meta-analysis showed that the summary RR with sunitinib versus control for all-grade hypertension was 3.44 (95% CI: 0.62 to 19.15, p = 0.16) in these cancer patients using the random-effects model (Test of heterogeneity: Q = 16.230, p < 0.001, I2=93.839); while the summary RR for high-grade hypertension was 22.72 (95% CI: 4.48 to 115.3, p < 0.001) using the fixed-effects model (Test of heterogeneity: Q = 0.524, p = 0.469, I2<0.001). Thus, on the whole sunitinib is associated with a significantly increased risk of high-grade not all-grade hypertension when compared with controls in these cancer patients.
Risk of creatinine increase
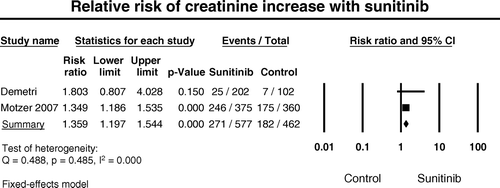
In order to understand the mechanism of sunitinib-associated hypertension, we also investigated whether sunitinib contributes to the development of renal dysfunction in these cancer patients with many other confounding factors for such as diabetes, nephrotoxic medications and contrast. Data for creatinine elevation with appropriate controls are only available for the two randomized controlled trials in patients with GIST and RCC () Citation[6], Citation[9]. The incidence of all-grade creatine increase was 65.6% (95% CI: 60.6–70.2%) among the patients with RCC receiving sunitinib, and was 12.4% (95%CI: 8.5–17.7%) among patients with GIST. Meta-analysis of relative risk (RR) associated with sunitinib in comparison with controls was performed. Sunitinib was found to be associated with a significant increased risk for all-grade creatinine increase. As shown in , the summary RR was 1.36 with sunitinib versus control (95% CI: 1.20 to 1.54, p < 0.001). However, there was also a variation in RR between these two types of cancer. For patients with GIST, RR was 1.80 (95% CI: 0.81 to 4.2, p = 0.15), while for patients with RCC, RR was 1.35 (95% CI: 1.19 to 1.54, p < 0.001).
Figure 2. Relative risk of renal dysfunction associated with sunitinib versus control. The summary RR is calculated using a random-effects model. RR and 95% confidence intervals for each study and the final combined result are displayed numerically on the left and graphically as a forest plot on the right. Under study name, the first author's name was used to represent each trial. The size of the squares is directly proportional to the amount of information available. For individual trials: filled-in square, incidence; lines, 95% confidence interval; diamond plot, overall results of the included trials.
Discussion
Our study has evaluated the risk of hypertension in cancer patients receiving sunitinib by the meta-analysis of published prospective clinical trials. This study demonstrated a high incidence of hypertension (all-grade: 22.5%, 95% CI: 19.5–25.9%; high-grade: 6.8%, 95% CI: 5.3–8.8%) associated with sunitinib in cancer patients. The majority of hypertension associated with sunitinib is grade I or II. The significance of moderate hypertension in cancer patients treated with sunitinib is less clear. Since patients with cancer are surviving longer, improving the quality of life by reducing complications is an important issue. It is accepted that hypertension is an independent risk factor for both renal and cardiovascular events Citation[24], Citation[25]. Therefore, it is important that mild to moderate hypertension induced by sunitinib be recognized and managed appropriately. Reduction of left ventricular (LV) systolic function has been reported in RCC patients receiving sunitinib therapy Citation[11]. A recent retrospective study of cardiovascular events in 75 patients with GIST showed that 8% with congestive heart failure, and 28% with LVEF reduction of more than 10% Citation[10]. Although hypertension can lead to LV dysfunction, it is not clear whether LV dysfunction in this case is secondary to hypertension or direct myocardial injury due to the medication Citation[10]. Anyway, it is possible that LV dysfunction may be at least exacerbated by hypertension.
Severe hypertension is not infrequent with the use of sunitinib (high-grade: 6.8%, 95% CI: 5.3–8.8%). Our study also showed that sunitinib is associated with a significantly increased risk of high-grade hypertension in comparison with controls (RR = 22.72, 95% CI: 4.48 to 115.29, p < 0.001) (). According to the package insert, severe hypertension (>200 mmHg systolic or 110 mmHg diastolic) occurred in 10/169 RCC patients (6%), 8/202 GIST patients on sunitinib (4%), and 1/102 GIST patients on placebo (1%). The significance of severe hypertension is evident because of its cardiovascular complications. In addition, similar to other anti-VEGF therapy, severe hypertension from sunitinib may lead to reversible posterior leukoencephalopathy Citation[26], Citation[27].
In addition to sunitinib, several other angiogenesis inhibitors such as bevacizumab and sorafenib have been associated with hypertension Citation[28]. The absolute risk of hypertension is substantial among these angiogenesis inhibitors. The incidence of hypertension associated with high-dose bevacizumab, a monoclonal antibody against VEGF (vascular endothelial growth factor), is 25.4% (95%CI: 21.3–30.1%) by a meta-analysis Citation[29], Citation[30]; the incidence of hypertension associated with sorafenib, another multi-tyrosine kinase inhibitor with activity against VEGFR, is 23.4% (95% CI: 16.0–32.9%) Citation[29]. Our study here demonstrated that the incidence of hypertension with sunitinib is 22.5% (95% CI: 19.5–25.9%).Therefore, it appears that the incidences of hypertension associated with these angiogenesis are remarkably similar. The mechanism of hypertension associated with these angiogenesis inhibitors is not clear. It may be directly related to their antagonistic effect on VEGF signal pathways Citation[31], resulting in a reduced density of micro-vessels, endothelial dysfunction associated with reduced nitric oxide production and increased oxidase stress, and changes in neurohormonal factors. Significant reduction of microvascular density or “rarefaction” has been observed in cancer patients treated with bevacizumab recently Citation[32]. A small study by Veronese et al. showed that the role of neurohumoral factors in the sorafenib-induced hypertension may be limited Citation[33].
The risk of hypertension may vary substantially with tumor type. The absolute risk of developing hypertension is significantly higher in patients with RCC when compared with non-RCC cancers (25.9% versus 20.4%, RR 1.27, 95% CI: 1.13–1.43%, p < 0.001). This could be secondary to higher baseline blood pressure in patients with RCC than non-RCC. However, relative risk analysis from the two randomized controlled studies showed that the impact of sunitinib is more evident in RCC. RR was 8.20 (95% CI: 4.70 to 14.29) for patients with RCC Citation[6], and only 1.42 (95% CI: 0.81 to 4.2) for patients with GIST Citation[9]. One possible explanation is that patients with RCC may have higher VEGF level than non-RCC patients, and the resulting overall anti-VEGF effect of sunitinib may be more evident. In fact, 90% of patients with RCC have the clear cell type in which VEGF expression is significantly increased due to loss-of function mutations in Von Hippel-Lindau (VHL). Alternatively, patients with RCC may have reduced renal function due to prior nephrectomies, and thus may have reduced excretion of sunitinib level leading to increased sunitinib exposure or directly contribute to the development of hypertension. Indeed, majority of patients with RCC in these trials had nephrectomies before receiving sunitinib.
The risk of hypertension is also associated with the dosing schedule of sunitinib. Our results revealed that there is a significantly increased risk of developing hypertension when being treated with the continuous daily dosing in comparison with the intermittent dosing schedule (RR 1.32, 95% CI: 1.18–1.48, p < 0.001). The reason for this difference is unclear. Intermittent dosing has higher drug concentration in tissues but shorter duration that may have less profound impact on the systematic vasculature than continuous dosing with somewhat lower concentrations but prolonged exposure. It is possible that the two off weeks may allow better vascular endothelial recovery from the damage of sunitinib than the continuous daily dosing.
The risk of hypertension may be related to renal dysfunction from suninitib. Our study also demonstrated that sunitinib is associated with a high incidence of renal dysfunction (65.6% among the patients with RCC and 12.4% among the patients with GIST) and increased risk in comparison with controls (RR 1.359, 95% CI: 1.197, 1.544; p < 0.001) (). One possible explanation is that it may be related to poor renal reserve. Over 90% of patients with RCC in the clinical trials treated with sunitinib had prior nephrectomy. Thus, these patients had lower nephron mass and lower glomerular filtration rate (GFR). These patients may be more sensitive to renal toxins. We hypothesize that the incidence of renal dysfunction may be underestimated. Creatinine is not a sensitive test for monitoring kidney function. In mild renal dysfunction, an initial decline in GFR may lead to only a slight increase (0.1 to 0.2 mg/dL) in the serum creatinine level because of an increase in proximal tubular creatinine secretion. The net effect is that patients with a true GFR as low as 60 to 80 mL/min may still have serum creatinine ≤1.0 mg/dl Citation[34]. Current NCI-CTC toxicity grading criteria may underestimate worsening renal function if a patient has normal GFR prior to sunitinib treatment. Although it is not mentioned in the package insert, renal function should be monitored closely, especially in patients already have reduced GFR.
The clinical significance of sunitinib-associated renal dysfunction has not been evaluated and poorly understood. It is well-known that renal disease is an independent risk factor for cardiovascular disease. It is possible that the sunitinib-associated renal dysfunction may contribute to the development of hypertension in these patients. Due to the increasing use of this medication, close post market surveillance of renal function may be important. The etiology of sunitinib-associated renal dysfunction is not known. It is possible that uncontrolled hypertension may cause renal injury. However, kidney injury may result from a direct toxicity of sunitinib to glomeruli and renal tubules due to its anti-VEGFR effect because VEGF is expressed both in podocytes in the glomerulus and tubular cells. In animal model, decreased VEGF expression in podocytes lead to significant glomerulopathy Citation[35].
Management of sunitinib-associated hypertension remains controversial. According to manufacturer recommendations, blood pressure should be monitored. However, no specific instructions were given for monitoring frequency. Hypertension secondary to sorafenib, a similar tyrosine kinase inhibitor, occurred early in the course of treatment, and blood pressure is recommended to be monitored weekly during the first 6 weeks of sorafenib therapy. Thus, it is reasonable to monitor blood pressure in a similar fashion for sunitinib. In case of severe or persistent hypertension, despite institution of antihypertensive therapy, temporary or permanent discontinuation of sunitinib should be considered Citation[36]. In most patients, hypertension can be controlled with standard anti-hypertensive medications. However, the biological effect of these antihypertensive medications on angiogenesis and its implications needs further investigation. Both enalapril and candesartan (ACE inhibitor and ARB, respectively) may inhibit myocardial angiogenesis induced by VEGF Citation[37]. In addition, candesartan may have antiangiogenesis impact as shown in a xenograft model of bladder cancer Citation[38]. On the other hand, nifedipine (a calcium channel blocker) was shown to induce VEGF secretion Citation[39]. Thus, it is possible that some antihypertensive medications may be more effective in treating anti-VEGF associated hypertension, and have less toxicity in conjunction with sunitinib. In addition, certain antihypertensive medications may interfere with the efficacy of sunitinib by modulating VEGF expression and reducing its antiangiogenesis effect. Further studies is needed to address these issues.
Another concern is drug-drug interactions. Sunitinib is metabolized primarily in the liver, undergoing oxidative metabolism mediated by the cytochrome p450 enzyme system, mainly by CYP3A4 Citation[36]. Therefore, the metabolism of sunitinib may be potentially altered by certain antihypertensive agents which are inhibitors of CYP3A4. For example, nondihydropyridine calcium channel blockers such as verapamil and diltiazem have the property of CYP3A4 inhibitors, and may significantly increase the plasma concentration of sunitinib. Concurrent administration of sunitinib with the strong CYP3A4 inhibitor ketoconazole, resulted in 49 and 51% increases in the combined Cmax (peak concentration) and AUC (area-under-curve) values of sunitinib and its primary active metabolite in healthy volunteers after a single dose sunitinib Citation[11]. Thus, selection of an alternate concomitant medication with no or minimal enzyme inhibition potential is suggested. A dose reduction for sunitinib should be considered when it must be co-administered with strong CYP3A4 inhibitors. In the absence of available data from clinical studies, dihydropyridines such as amlodipine and nifedipine may be the preferred class of calcium channel blockers. Nondihydropyridine calcium channel blockers should be used cautiously in conjunction with sunitinib. Alternatively, ACE inhibitors or angiotensin-receptor blockers are reasonable choices with the additional benefit of improving endothelial function and microcirculatory density Citation[40]. Other anti-hypertensive medications such as diuretics, alpha blocker, beta-blockers, which have been used in the setting of bevacizumab-associated hypertension, Citation[41] and may be considered for use in this setting. Additional studies directed at uncovering the mechanism of anti-angiogenesis therapy induced hypertension are required to guide therapy.
The results described here are limited by individual clinical trials that were selected for the meta-analysis. Even though we showed that no statistically significant difference for the overall risk of all-grade hypertension in cancer patients was found between sunitinib and controls (RR = 3.44, 95% CI: 0.619–19.15, p = 0.158), this is largely due to a limitation of available randomized studies. These trials may also underestimate the incidence of hypertension and renal dysfunction associated with sunitinib, which used the National Cancer Institute Common Toxicity Criteria Version II or III Citation[12]. In these two versions of toxicity criteria, patients were considered hypertensive only if diastolic pressure increased > 20 mmHg, or BP was > 150/100 mmHg. This grading criteria likely underestimates the incidence of hypertension according to the standard criteria for the diagnosis of hypertension (140/90 mmHg). However, the incidences of hypertension were relatively consistent among all the trials selected. Moreover, creatinine was used as the marker for renal dysfunction. As already well established, creatinine is not a sensitive marker for renal function changes, particularly in cases of mild to moderate renal dysfunction. Second, baseline hypertension was not mentioned in these trials. We have minimized the likelihood of bias by calculating relative risk using randomized controlled clinical trials, with direct comparison with and without sunitinib before the analysis. Third, sample size may affect our analysis. Even though we were not able to detect significant difference in hypertension between intermittent and continuous dosing of sunitinib (RR 1.37, p = 0.06), this result may be limited by the small sample size of patients receiving continuous dosing of sunitinib (n = 107). Finally, patients in this study were a selected group of patients involved in clinical trials with metastatic cancers and adequate organ function. The results were observed in academic centers, and may not entirely apply to patients treated in the community.
In conclusion, this study demonstrates that sunitinib, a multi-tyrosine kinase inhibitor with anti-angiogenesis effect, is associated with a significant risk of developing hypertension and renal dysfunction in a group of patients with metastatic cancers. The risk of hypertension varies with tumor types and sunitinib dosing schedule, with higher risk for patients with RCC and continuous daily dosing. Further studies will be needed to address other possible risk factors such as gender, age, tobacco smoking, and ECOG performance status. As sunitinib is increasingly used in cancer patients, early detection and effective management of hypertension may allow for more extensive and safer use. The hypertension and renal side effects of sunitinib may require thorough post-marketing surveillance and reporting. Future studies will be needed to focus on uncovering the mechanisms and appropriate treatment of sunitinib-induced hypertension.
Acknowledgements
S.W. is supported by the Research Foundation of SUNY, and is a speaker for Pfizer Inc., and has received honoraria from Onyx Pharmaceuticals.
References
- Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2003; 2: 471–8
- O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 2003; 101: 3597–605
- Osusky KL, Hallahan DE, Fu A, Ye F, Shyr Y, Geng L. The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis 2004; 7: 225–33
- Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 2003; 20: 757–66
- Schueneman AJ, Himmelfarb E, Geng L, et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Res 2003; 63: 4009–16
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New Engl J Med 2007; 356: 115–24
- Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006; 24: 16–24
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006; 295: 2516–24
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006; 368: 1329–38
- Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007; 370: 2011–9
- Pfizer. Sutent Prescribing Information, updated on 2/07, available at http://www.pfizer.com/pfizer/download/uspi_sutent.pdf. 2006.
- NCI, Cancer Therapy Evaluation Program. CTC v 2.0 and common terminology criteria for adverse events criteria V3.0 (CTCAE). Available at http://ctep.cancer.gov/reporting/ctc.html. 2006.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127: 820–6
- Gore ME, Oudard S, Bjarnason G, Castellano D, Szczylik C, Mainwaring PN, et al. Sunitinib in Metastatic Renal Cell Carcinoma (mRCC): Preliminary assessment in an expanded access trial with subpopulation analysis. In: 43rd ASCO Annual Meeting; 2007 June 20, 2007; Chicago, IL; 2007. p. 237s.
- Reichardt P, Ruka W, Seddon B, Baum CM, Demetri GD. Subpopulation analyses in a worldwide treatment-use trial of sunitinib (SU) in GIST patients (pts) with resistance or intolerance to prior imatinib (IM) therapy. In: 43rd ASCO Annual Meeting; 2007 June 20, 2007; Chicago, IL; 2007. p. 550s.
- Bang Y, Kang W, Boku N, Chung H, Lanzalone S, Lechuga MJ et al. Sunitinib as second-line treatment for advanced gastric cancer: Preliminary results from a Phase II study. In: 43rd ASCO Annual Meeting; 2007 June 20, 2007; Chicago, IL; 2007. p. 223s.
- Brahmer JR, Novello S, Rosell R, Belani CP, Atkins JN, Gillenwater HH et al. Efficay and safety of continuous daily sunitinib dosing in previously treated advanced non-small cell lung cancer (NSCLC): Results from a Phase II study. In: 43rd ASCO Annual Meeting; 2007 June 20, 2007; Chicago, IL; 2007. p. 395s.
- George S, Casali PG, LeCesne A, Morgan JA, Pokela J, Quigley MT et al Continuous daily dosing of sunitinib malate compares favorably with intermittent dosing in patients with advanced GIST. In: 43rd ASCO Annual Meeting; 2007 June 20, 2007; Chicago, IL; 2007. p. 548s.
- Iasonos A, Riches J, Bajorin DF. Phase II study of sunitinib in patients with repalpsed or refractory urothelial carcinoma. In: 43rd ASCO Annual Meeting; 2007 June 20, 2007; Chicago, IL; 2007. p. 254s.
- Rini BI, George DJ, Michaelson MD, et al. Efficacy and safety of sunitinib Malate (SU11248, Sutent) in bevacizumab-refractory metastatic renal cell carcinoma. In: ASCO Annual meeting; 2006; Atlanta, GA; 2006. p. 222s, Abstract # 4522.
- Socinski MA, Novello S, Sanchez JM et al Efficacy and safety of sunitinib in previously treated advanced non-small cell lung cancer (NSCLC): Preliminary results of a multicenter phase II trial. ASCO Annual Meeting Proceedings 2006;24: 364s, Abstract # 7001.
- Srinivas S, Gillessen S, Hamenberg U, De Mulder PH, Fountzilas G, Vogelzang N et al Continuous daily administration of sunitinib in patients with cytokine-refractory mestatic renal cell carcinoma: Updated results. In: 43rd ASCO Annual Meeting; 2007 June 20, 2007; Chicago, IL; 2007. p. 244s.
- Kaplan NM, Opie LH. Controversies in hypertension. Lancet 2006; 367: 168–76
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The modification of diet in renal disease study. Ann Internal Med 1995; 123: 754–62
- Kapiteijn E, Brand A, Kroep J, Gelderblom H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol 2007; 18: 1745–7
- Martin G, Bellido L, Cruz JJ. Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J Clin Oncol 2007; 25: 3559
- Zhu X, Perazella MA. Anti-vascular endotehlial growth factor (VEGF) therapy: A new cause of hypertension. Hypertens Rev 2007;accepted.
- Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in cancer patients. Lancet Oncol 2008; (in press).
- Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis 2007; 49: 186–93
- Sica DA. Angiogenesis inhibitors and hypertension: An emerging issue. J Clin Oncol 2006; 24: 1329–31
- Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol 2007.
- Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol 2006; 24: 1363–9
- Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Inter 1985; 28: 830–8
- Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003; 111: 707–16
- Bayer. NEXAVAR Prescribing Information, 8/06, available at http://www.nexavar.com/wt/page/index. 2006.
- Siddiqui AJ, Mansson-Broberg A, Gustafsson T, et al. Antagonism of the renin-angiotensin system can counteract cardiac angiogenic vascular endothelial growth factor gene therapy and myocardial angiogenesis in the normal heart. Am J Hypertens 2005; 18: 1347–52
- Kosugi M, Miyajima A, Kikuchi E, Horiguchi Y, Murai M. Angiotensin II type 1 receptor antagonist candesartan as an angiogenic inhibitor in a xenograft model of bladder cancer. Clin Cancer Res 2006; 12: 2888–93
- Miura S, Fujino M, Matsuo Y, Tanigawa H, Saku K. Nifedipine-induced vascular endothelial growth factor secretion from coronary smooth muscle cells promotes endothelial tube formation via the kinase insert domain-containing receptor/fetal liver kinase-1/NO pathway. Hypertens Res 2005; 28: 147–53
- Agabiti-Rosei E. Structural and functional changes of the microcirculation in hypertension: influence of pharmacological therapy. Drugs 2003; 63: 19–29
- Mares JE, Worah S, Mathew SV, et al. Increased rates of hypertension (HTN) among patients with advanced carcinoid treated with bevacizumab. The 41st Annual Meeting of the American Society of Clinical Oncology (ASCO) 2005: Abstract No: 4087.