Abstract
Background. The MammoSite® radiotherapy system is an alternative treatment option for patients with early-stage breast cancer to overcome the longer schedules associated with external beam radiation therapy. The device is placed inside the breast surgical cavity and inflated with a combination of saline and radiographic contrast to completely fill the cavity. The treatment schedule for the MammoSite monotherapy is 34 Gy delivered in 10 fractions at 1.0 cm from the balloon surface with a minimum of 6 hours between fractions on the same day. Material and methods. This review article presents the advantages, disadvantages, uncertainties and clinical outcomes associated with the MammoSite brachytherapy (MSB). Results. Potential advantages of MSB are: high localised dose with rapid falloff for normal tissue sparing, minimum delay between surgery and RT, catheter moves with breast, improved local control, no exposure to staff, likely side-effects reduction and potential cost/time saving (e.g. for country patients). The optimal cosmetic results depend on the balloon-to-skin distance. Good-to-excellent cosmetic results are achieved for patients with balloon-skin spacing of ≥7 mm. There have been very few published data regarding the long term tumour control and cosmesis associated with the MSB. The available data on the local control achieved with the MSB were comparable with other accelerated partial breast irradiation techniques. The contrast medium inside the balloon causes dose reduction at the prescription point. Current brachytherapy treatment planning systems (BTPS) do not take into account the increased photon attenuation due to high Z of contrast. Some BTPS predicted up to 10% higher dose near the balloon surface compared with Monte Carlo calculations using various contrast concentrations (5–25%). Conclusion. Initial clinical results have shown that the MammoSite device could be used as a sole radiation treatment for selected patients with early stage breast cancer providing good local control, minimal complication rate and excellent cosmesis.
Breast conserving therapy (BCT) has become an accepted alternative to mastectomy for treatment of patients with early stage breast cancer Citation[1], Citation[2]. This technique has been reported to result in local control rates equivalent to that of mastectomy in patients with early stage breast cancer Citation[3–5]. It consists of the removal of breast tumour surgically (lumpectomy) followed by 5–7 weeks of daily whole breast irradiation course Citation[6], Citation[7]. During the standard radiotherapy procedure a total dose of 45–50 Gy is delivered to the entire breast Citation[8], Citation[9]. However, the disadvantage of this approach is its prolonged time which may present obstacles for many patients, especially the elderly and those who live far away from the radiotherapy facility Citation[10].
To tackle this problem, accelerated partial breast irradiation (APBI) has been introduced as an alternative treatment method for patients with early stage breast cancer. One form of APBI uses interstitial brachytherapy (IB) implant technique Citation[1], Citation[9], Citation[11–13]. With this technique, it became possible to give a large dose per fraction to a limited portion of breast tissue adjacent to the lumpectomy cavity in a shorter treatment time. However, IB is not trivial to deliver and is only provided by a limited number of cancer centres Citation[14–16]. Moreover, the treatment planning with this technique is time-consuming and requires experienced staff Citation[17].
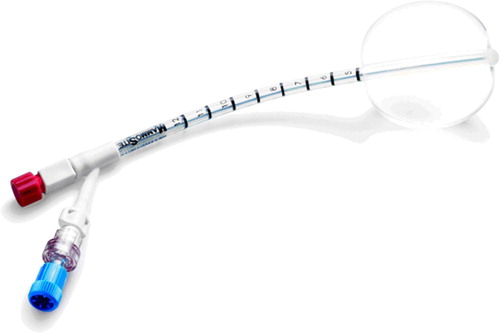
In response, a new approach to breast brachytherapy has been developed in recent years Citation[18]. This novel technique uses the MammoSite® brachytherapy (MSB) system (Cytyc, Marlborough, MA) as a sole radiation treatment for patients with early stage breast cancer following lumpectomy. It can also be used as a boost in conjunction with external beam radiotherapy (EBRT). It consists of a small balloon connected to an inflation channel and a catheter for the passage of a high dose rate brachytherapy source (Ir-192) shown in . The device is placed in the lumpectomy cavity during or following breast surgery Citation[18]. The MammoSite balloon is inflated with the sterile saline containing a small amount of radiographic contrast to a size that both completely fills the cavity and ensures conformance of the tissue to the balloon Citation[19], Citation[20]. Computed Tomography (CT) scan is obtained to assess balloon conformance to the lumpectomy cavity and to determine the distance from the surface of the balloon to the skin, the symmetry, diameter of the inflated balloon, the planning target volume and the dose distribution Citation[18]. The treatment with the MammoSite device is generally 34 Gy delivered in 10 fractions (3.4 Gy/fraction twice daily) at 1.0 cm from the balloon surface with a minimum of 6 hours between fractions on the same day Citation[18], Citation[21]. There are recommendations regarding the patient selection for the MammoSite treatment. Generally, it is used for treatment of patients diagnosed with ductal carcinoma in situ (DCIS), invasive ductal carcinoma (IDC) and a primary tumour of size ≤3 cm.
The MammoSite brachytherapy device has been commercially available since 2002. The first published study on the applicator use has demonstrated that both device insertion and performance are simple and efficient Citation[18]. Also, the treatment planning time may be decreased to 25 minutes Citation[22]. Other advantages of the MSB technique have included better dose homogeneity, sparing of normal tissues, less radiation exposure to the staff and potential reduction in acute and late side effects. In the past 5 years a few studies investigated various aspects of the MammoSite technique, including the volume of breast tissue treated compared to IB and EBRT techniques. Others have described the dose perturbation caused by the radiographic contrast in the balloon. Recently, clinical results have appeared reporting on the cosmetic outcomes and tumour control for patients treated with the MammoSite as a sole radiation method. This review article summarizes the advantages, disadvantages, uncertainties and clinical outcomes associated with the MSB radiation system.
Dosimetry of MammoSite
Limited publications have evaluated the dosimetry achieved with the MSB Citation[17], Citation[18], Citation[23–25]. Edmundson et al.Citation[18] reported the first study on the MSB dose distribution. Eight patients were treated and a CT scan of each patient was used to determine the treatment parameters, namely the balloon radius, balloon symmetry, the planning target volume (PTV) and the distance from the balloon surface to the skin. The MammoSite dosimetric data was analysed and compared to a similar group of patients treated with IB at the same institution. The mean PTV of the MammoSite patients was 112.1 cm3 compared with 98.3 cm3 for IB patients. The MSB provided better PTV coverage compared to that achieved with IB. The D90, the minimum dose to 90% of the PTV, was calculated for both techniques. The mean values were 90.0% and 69.8% of the total dose for the MammoSite and the IB methods respectively. The MSB resulted in significant improvement in dose distribution. However, the MammoSite device with the balloon radius larger than 3.0 cm was found to result in longer treatment times and to give rise to higher doses to the normal structures, such as the heart and the lungs. As a result, a cavity with volume of 50 cm3 represents an upper limit on the size of the cavity that can be treated with the MammoSite balloon Citation[18].
Dickler et al. Citation[23] presented results on the volume of breast tissue treated with the MammoSite applicator. The study included 21 patients with early stage breast cancer. The volume of the treated breast tissue was constructed on the basis of scans of the lumpectomy cavity of each patient both with and without the inflated balloon. It was found that the volume of breast tissue treated by the MammoSite device was equal to the volume encompassed by a margin of 1.6 cm around the empty lumpectomy cavity. This is comparable with IB which treats a 1–2 cm of tissue margin around the lumpectomy cavity.
Major et al. Citation[17] reported a dosimetric comparison between treatment plans of IB and MSB patients. The comparison included 17 and 24 patients treated with IB and MSB respectively. The average volume and dose parameters for IB and MSB techniques are listed in . The plans for all patients were based on CT images, and the dose distributions were evaluated using dose volume histograms (DVHs). The average volume of PTV was considerably higher for MSB than IB patients at 109.6 cm3 and 63.4 cm3, respectively. The small volume of the PTV for IB patients is due to the safety margin of 1.0 cm used around the lumpectomy cavity. The volume of the PTV receiving twice the prescribed dose (V200%) was less for the MSB patients. The dose distribution was much more conformal in the MSB than IB. However, the definitions of the PTV were different in the IB and MSB groups. In IB an individual margin can be used in different directions, while in MSB a uniform margin of 1.0 cm from the balloon surface is used. The doses to the heart were not significantly different between the two groups. But the maximum point dose to the lung was higher in MSB plans compared to IB plans. Overall the MSB has produced acceptable dosimetry, but longer follow-up time was recommended to evaluate its clinical efficacy.
Table I. Average volume and dose parameters for IB and MSB patients [17].
Weed et al.Citation[24] reported the first single-institutional dosimetric comparison of patients treated with three APBI techniques: IB, MSB and 3D Conformal Radiation Therapy (3D-CRT). DVHs were used to evaluate the dose coverage of the PTV and the doses to normal structures including the ipsilateral breast, ipsilateral lung, and heart. A summary of patient dose and volume information for all three techniques is presented in . Coverage of the PTV varied according to the technique used and the margin drawn around the lumpectomy cavity. The 3D-CRT offered the best PTV coverage. The average PTV volume for the 3D-CRT patients was twice larger than the average PTV for the MSB patients and slightly larger than the average PTV for the IB patients. The percentage of the PTV receiving 100% of the prescribed dose was 58% in the IB method compared with 76% and 94% with the MSB and 3D-CRT techniques respectively. The percentage of the PTV receiving 90% of the prescribed dose was 68%, 91% and 100% for the IB, the MSB and 3D-CRT techniques respectively.
Table II. Patient characteristics including total dose, volume of lumpectomy cavity, volume of ipsilateral breast, volume of ipsilateral lung and heart volume [24].
Both brachytherapy techniques delivered significantly less dose to the normal breast tissue as compared with the 3D-CRT Citation[24]. For example, the percentage volume of the ipsilateral breast receiving 100% of the prescribed dose was 5%, 10% and 24% for the MSB, IB and 3D-CRT patients, respectively. However, both brachytherapy methods treated higher portion of the breast volume above 115% of the prescribed dose compared with the 3D-CRT. For instance, the MSB and IB treated 4% and 5% of the breast volume to 115% of the prescribed dose, respectively while 0% of the breast volume received 115% of the prescribed dose with the 3D-CRT. All three techniques resulted in small dose to normal structures. The average volume of the ipsilateral lung receiving 10% and 20% of the prescribed dose was 9% and 5% for 3D-CRT compared with 3% and 0% for IB and 4% and 0% for the MSB patients. The average volume of the heart receiving 10% of the prescribed dose was 1% for each of the three techniques.
The above study concluded that the MammoSite device consistently provided better dose coverage of the PTV as compared to IB. But the 3D-CRT offered better coverage of the PTV in comparison to either of the brachytherapy techniques. However, this resulted in a higher dose to the normal breast tissues and lung. As a result, the optimal partial breast irradiation technique is a clinical decision. The shortcoming of the study is that it does not compare dosimetry in identical patient data sets. The patients representing the three different treatment groups were different which can make the dosimetric comparison less straightforward.
Khan et al. Citation[25] presented dosimetric comparison of three different methods of APBI: The MSB, 3D-CRT and Intensity Modulated Radiation Therapy (IMRT). It consisted of 15 patients who underwent BCT. With the use of CT images, plans were developed for each patient using each of the three techniques. A summary of the dosimetric results for the various techniques is summarized in . The volume of the PTV was smaller in MSB plans than in 3D-CRT and IMRT plans. This is attributed to the 2.5 cm margin which was used around the lumpectomy cavity to generate the PTV for both 3D-CRT and IMRT methods. All three groups had low volume of the lung receiving 30% of the prescribed dose, but the IMRT patients had the lowest value. An interesting result from the study is that the MSB technique resulted in significantly higher dose to the heart (V5) as compared to both 3D-CRT and IMRT method for the ten patients with left-sided tumours. This is because, as opposed to MSB treatment planning, the heart is contoured as an organ at risk in both 3D-CRT and IMRT plans and the dose delivered can thus be constrained and optimized. Both 3D-CRT and IMRT methods have the advantage of not requiring a placement of foreign object inside the patient. However, the difficulty of identifying the cavity in certain patients, respiratory motion and patient setup may be of concern when using 3D-CRT and IMRT techniques. On the other hand, these issues are not significant with the use of the MSB. It was concluded that the choice of optimal PBI method is complex and requires both the doctor's decision and suitable patient selection.
Table III. Dosimetric comparison of 3 different APBI techniques [25].
Balloon contrast concentration
Once in place, the MammoSite device is inflated to a size that fills the surgical cavity using saline and an amount of radiographic contrast. CT scans are then obtained and the treatment plan is developed. Current available BTPS (e.g Plato, Nucletron) perform dosimetry calculations according to the recommendations of the AAPM TG-43 protocol Citation[26]. This protocol assumes that the high dose rate brachytherapy source is located in water medium. Since the contrast medium contains elements with high atomic number (iodine, Z = 53), the balloon is no longer tissue or water equivalent. Thus, the BTPS do not accurately predict the dose at the balloon surface. Several studies have used Monte Carlo (MC) simulations to determine the dose reduction factor corresponding to a range of contrast concentrations Citation[19], Citation[20], Citation[27–29]. In , a summary of the dose reduction factors at 1 cm from the balloon surface for balloon radii of 2 & 3 cm and several contrast concentrations determined in different studies is presented.
Table IV. Dose perturbation factors using MC calculations at 1 cm from the balloon surface for various balloon radii and contrast concentration percentages [19,20].
Mitra et al. Citation[27] performed measurements of the dose perturbations resulting from the use of various contrast concentrations (5–25%). The BTPS predicted about 9% higher dose near the balloon surface compared with measured values. This was attributed to the lack of inhomogeneity corrections in the BTPS. Ye et al. Citation[28] performed MC calculations and found that a 25% contrast concentrations inside the balloon resulted in up to 5% dose reduction at the surface of the balloon. Other investigators reported that, with a 4% contrast concentration, the BTPS overestimated the dose at the balloon surface by about 10% as compared with MC calculations Citation[29]. These studies have demonstrated that the dose rate reduction depends on the concentration of contrast material. It has been suggested that limiting the contrast concentration to 10% would ensure less than 3% reduction in the prescription dose, regardless of the balloon size Citation[19].
MammoSite and cosmetic outcomes
The two most important factors for achieving treatment effectiveness and optimal cosmetic results are balloon cavity conformance and skin-to-balloon surface distance. The former is essential in order to achieve good dose homogeneity within the PTV. The latter is for restraining the maximum skin dose. A minimum distance between the balloon and the skin of 5 mm was chosen to keep the skin dose less than 150% of the prescribed dose Citation[18], Citation[30]. However, recent publications recommend a balloon-to-skin distance larger than 7 mm to achieve excellent cosmetic results Citation[31], Citation[32]. The clinical results of recent publications using the MSB as sole radiation treatment following lumpectomy are summarized in . For example, Dragun et al. Citation[32] reported excellent cosmetic results in 68.9%, good in 21.1%, fair in 8.9% and poor in 1.1% of 100 patients treated with the MammoSite device at a median follow-up of 2 years. No breast tumour recurrence was reported. Overall, the excellent cosmetic results were associated with a balloon-to-skin distance larger than 7 mm.
Table V. Clinical results of cosmetic outcomes and tumour recurrence using the MSB.
Jeruss et al. Citation[42] reported clinical results of 169 patients who had breast cancer treatment with the MammoSite balloon device. The follow-up data was available for 158 patients. The average length of the follow-up was more than seven months. Patients with the balloon-to-skin distance ≥ 7 mm had the best cosmetic results and less skin damage. These findings were confirmed using data on 43 patients who had a follow-up period of at least one year. No patient in the study has experienced a recurrence of the disease.
Long term follow-up data
There have been very few published data regarding the long term tumour control and cosmesis associated with the MSB. Chao et al. Citation[44] presented the results of eighty patients treated with the MammoSite applicator. Up to 88.2% of patients were found to have good/excellent cosmetic results at 3-year follow-up. Ipsilateral breast-tumour recurrence occurred in two patients (2.5%) Both recurrences occurred within 12 months after completion of the MammoSite treatment. The 3-year disease-free survival rate for all patients was 95.8%. At 24 months follow-up, an increased balloon-to-skin spacing ≥ 7 mm was associated with high occurrence of good-excellent cosmetic results, highlighting the importance of the distance from the balloon surface to skin to avoid excessive dose to the skin. The 3-year follow-up results demonstrated that the MammoSite treatment outcomes were similar to those observed with IB at the same follow-up length.
Chen et al. Citation[45] reported 7.1% of treatment failures among 70 patients treated with the MammoSite technique at a median follow-up time of 26.1 months. The tumour recurrence for one case was directly adjacent to surgical bed and three cases were at more than 2 cm away from the original surgical bed. Benitez et al. Citation[46] presented the results of 5.5-year follow-up of 36 patients treated with the MSB. The cosmetic outcomes were good /excellent in 83.3% of the followed patients. There were no local recurrences at either the tumour bed or elsewhere in the breast for all 36 patients. The study concluded that the 5-year local recurrence using the MammoSite balloon in carefully selected patients was comparable to the 5-year data achieved with IB and EBRT.
The available 3-5 year results can serve as an indicator of the adequacy of the MammoSite treatment method in terms of tumour control in comparison with other APBI techniques. However, further data is still needed to document its long term efficacy.
MammoSite treatment complications
Limited information is available in the literature regarding the toxicities associated with the MSB. Richards et al. Citation[35] reported the acute toxicities of 27 patients treated with the MammoSite device as the sole method of radiotherapy. The median follow-up was 11 months. No acute toxicities were reported during the 5 days of treatment however 25% of the patients developed bright erythema and patchy moist desquamation, 7% developed confluent moist desquamation within the first 4 weeks but healed by week 12 and 16% of the treated patients developed infections. Harper et al. Citation[39] assessed the acute complications of 37 patients on the day of the treatment completion and four weeks after the course of the treatment. As many as 93.3% of the patients were happy with the MammoSite therapy however 5.4% (2 patients) experienced Grade 2 toxicity, 2.7% (1 patient) experienced Grade 3 toxicity, 16.2% (6 patients) developed wound infections and 32.4% (12 patients) developed seromas. Sadeghi et al. Citation[43] examined the dose delivered to the skin and the surrounding tissues in 67 patients treated with the MSB. Fifty six percent of the patients experienced no skin reaction at a median follow-up of 13 months, 35% developed erythema but it disappeared over several weeks, 4 (6%) patients had dry desquamation and 2 (3%) patients experienced moist desquamation, and received skin dose greater than 4.10 Gy per fraction. Agawal et al. Citation[47] assessed acute toxicities in 100 patients treated with the MSB. Persistent seroma was observed in 26% of the patients with a follow-up of 6 months. The study concluded that the development of seroma may be attributed to the delivery of high radiation doses to the surgical cavity margin. Evans et al. Citation[48] reported the risk of development of seroma in 38 patients. Persistent seroma was seen in 68.4% of the patients with a follow-up of more than 6 months. Of the patients with persistent seroma, 46% experienced some pain in the breast during the follow-up period. However, only 19.2% of those patients had these symptoms persisted at their last follow-up (median of 15.8 months) examination. Overall, the results of the studies of the acute complications associated with the MammoSite method are encouraging but these observations need to be validated with more published clinical data.
Conclusions
Previous investigators have demonstrated that the MammoSite device insertion into the patient and treatment execution is easy and efficient. In the studies reported, coverage of the PTV varied according to the radiation technique used and the definition of adequate coverage used. However, in general the MSB device produced better coverage of the PTV compared to IB implants. The coverage of the PTV was better with 3D-CRT, resulting however in a higher dose to normal breast tissue than the MammoSite brachytherapy technique.
For imaging purposes, the MammoSite balloon is filled with saline and radiographic contrast. The amount of radiographic contrast varies from one study to another for several reasons including oncologist preference and institutional protocol. The presence of the contrast medium inside the balloon can cause reduction in the dose at the prescription point depending on its concentration. The BTPS currently in clinical use do not take into account the attenuation of the photons caused by the contrast medium. Some BTPS predicted up to 10% higher dose near the balloon surface compared with MC calculations for contrast concentrations between 5–25%. As a result the amount of contrast medium used inside the balloon should be minimized to avoid a potentially significant reduction in the delivered dose.
It appears that two factors may limit the use of the MSB device. The conformance of the balloon to the cavity is essential to assure appropriate dosimetry. Additionally, the distance between the balloon and the skin appears to be the most important factor for achieving optimal cosmetic results. Cosmetic results were good-to-excellent in most patients with balloon-skin spacing of ≥7 mm.
The endpoint of the MammoSite treatment technique as with any other radiotherapy modality is to achieve high tumour control probability and minimal normal tissue complications. Existing clinical findings have demonstrated highly acceptable outcomes with the MSB applicator regarding tumour control, acute complications and cosmetic results. The data coming from the published clinical trials with an average follow-up of 16 months have neither reported tumour recurrence nor normal tissue complications among the treated patients. This indicates that smaller PTV coverage in MSB does not represent a significant reduction in the treatment outcomes while offering better patient comfort (shorter treatment time) and cosmesis. The currently available long term data on the MSB tumour control were comparable with other APBI techniques. In conclusion, the MammoSite is a suitable technique for APBI. It offers good outcomes and shorter treatment schedules.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baglan KL, Martinez AA, Frazier RC, Kini VR, Kestin LL, Chen PY, et al. The use of high-dose-rate brachytherapy alone after lumpectomy in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 2001; 50: 1003–11
- Lawenda BD, Taghian AG, Kachinic LA, Hamdi H, Smith BL, Gadd MA, et al. Dose-volume analysis of radiotherapy for T1N0 invasive breast cancer treated by local excision and partial breast irradiation by low-dose-rate interstitial implant. Int J Radiat Oncol Biol Phys 2003; 56: 671–80
- Blichert-Toft M, Rose C, Anderson JA, Overgaard M, Axelsson CK, Andersen LW, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr 1992; 11: 19–25
- Fisher B, Anderson S, Redmond CK, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333: 1456–61
- Van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000; 92: 1143–50
- Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347: 567–75
- Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–32
- Bartelink H, Horiot JC, Poortmans H, Struikmans H, Van den Bogaert W, Barillot I, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med 2001; 345: 1378–87
- Polgar C, Sulyok Z, Fodor J, Orosz Z, Major T, Takacsi-Nagy Z, et al. Sole brachytherapy of the tumor bed after conservative surgery for T1 breast cancer: Five-year results of a phase I-II study and initial findings of a randomized phase III trial. J Surg Oncol 2002; 80: 121–8
- Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst 2000; 92: 269–71
- Vicini FA, Horwitz EM, Lacerna MD, Dmuchowski CF, Brown DM, White J, et al. Long-term outcome with interstitial brachytherapy in the management of patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 1997; 37: 845–52
- Vicini FA, Kestin LL, Edmundson GK, Jaffray DA, Wong JW, Kini VR, et al. Dose-volume analysis for quality assurance of interstitial brachytherapy for breast cancer. Int J Radiat Oncol Biol Phys 1999; 45: 803–10
- Vicini FA, Baglan KL, Kestin LL, Mitchell C, Chen PY, Frazier RC, et al. Accelerated treatment of breast cancer. J Clin Oncol 2001; 19: 1993–2001
- Fentiman IS, Poole C, Tong D, Winter PJ, Gregory WM, Mayles HM, et al. Inadequacy of iridium implant as sole radiation treatment for operable breast cancer. Eur J Cancer 1996; 32A: 608–11
- Vicini F, Kini VR, Chen P, Horwitz E, Gustafson G, Benitez P, et al. Irradiation of the tumor bed alone after lumpectomy in selected patients with early-stage breast cancer treated with breast conserving therapy. J Surg Oncol 1999; 70: 33–40
- Kestin LL, Jaffray DA, Edmundson GK, Martinez AA, Wong JW, Kini VR, et al. Improving the dosimetric coverage of interstitial high-dose-rate breast implants. Int J Radiat Oncol Biol Phys 2000; 46: 35–43
- Major, T, Niehoff, P, Kovacs, G, Fodor, J, Polgar. Dosimetric comparisons between high dose rate interstitial and MammoSite balloon brachytherapy for breast cancer. Radiother Oncol 2006;79:321–8. Epub 2006 May 26.
- Edmundson GK, Vicini FA, Chen PY, Mitchell C, Martinez AA. Dosimetric characteristics of the MammoSite RTS, a new breast brachytherapy applicator. Int J Radiat Oncol Biol Phys 2002; 52: 1132–9
- Kassas B, Mourtada F, Horton JL, Lane RG. Contrast effects on dosimetry of a partial breast irradiation system. Med Phys 2004; 31: 1976–9
- Kirk MC, Hsi WC, Chu JC, Niu H, Hu Z, Bernard D, et al. Dose perturbation induced by radiographic contrast inside brachytherapy balloon applicators. Med Phys 2004; 31: 1219–24
- Vicini FA, Kestin LL, Goldstein NS. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: A pathologic analysis. Int J Radiat Oncol Biol Phys 2004; 60: 722–30
- Borg M, Yeoh E, Bochner M, Butters J, Van Doorn T, Farshid G, et al. Feasibility study on the MammoSite in early-stage breast cancer: Initial experience. Australas Radiol 2007; 51: 53–61
- Dickler A, Kirk M, Choo J, His WC, Chu J, Dowlatshahi K, et al. Treatment volume and dose optimization of MammoSite breast brachytherapy applicator. Int J Radiat Oncol Biol Phys 2004; 59: 469–74
- Weed DW, Edmundson GK, Vicini FA, Chen PY, Martinez AA. Accelerated partial breast irradiation: A dosimetric comparison of three different techniques. Brachytherapy 2005; 4: 121–9
- Khan AJ, Kirk MC, Mehta PS, Seif NS, Griem KL, Bernard DA, et al. A dosimetric comparison of three-dimensional conformal, intensity-modulated radiation therapy, and MammoSite partial-breast irradiation. Brachytherapy 2006; 5: 183–8
- Nath R, Anderson LL, Luxton G, Weaver KA, Williamson JF, Meigooni AS. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys 1995; 22: 209–34
- Mitra RC, Cheng C, Das I. Dose perturbation due to contrast medium in MammoSite: Dosimetric investigation of dose calculation accuracy. Med Phys 2003; 30: 1388
- Ye SJ, Brezovich IA, Shen S, Kim S. Dose errors due to inhomogeneities in balloon catheter brachytherapy for breast cancer. Int J Radiat Oncol Biol Phys 2004; 60: 672–7
- Cheng CW, Mitra R, Li XA, Das IJ. Dose perturbations due to contrast medium and air in mammosite treatment: An experimental and Monte Carlo study. Med Phys 2005; 32: 2279–87
- Keisch M, Vicini F, Kuske RR, Hebert M, White J, Quiet C, et al. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 2003; 55: 289–93
- Niehoff, P, Polgar, C, Ostertag, H, Major, T, Sulyok, Z, Kimmig, B, , et al. Clinical experience with the MammoSite radiation therapy system for brachytherapy of breast cancer: Results from an international phase II trial. Radiother Oncol 2006;79:316–20. Epub 2006 Jun 14.
- Dragun, AE,Harper, JL, Jenrette, JM, Sinha, D, Cole, DJ. Predictors of cosmetic outcome following MammoSite breast brachytherapy: A single-institution experience of 100 patients with two years of follow-up. Int J Radiat Oncol Biol Phys 2007;68:354–8. Epub 2007 Mar 26.
- Keisch M, Vicini F, Kuske R, Hebert M, White J, Quiet C, et al. Two-year outcome with the MammoSite breast brachytherapy applicator: Factors associated with optimal cosmetic outcome when performing partial breast irradiation. Int J Radiat Oncol Biol Phys 2003; 57: S315
- Keisch M, Vicini F, Scroggins T, Hebert M, White J, Quiet C, et al. Thirty month results with the MammoSite breast brachytherapy applicator: Cosmesis, toxicity and local control in partial breast irradiation. Int J Radiat Oncol Biol Phys 2004; 60: S272
- Richards GM, Berson AM, Rescigno J, Sanghavi S, Siegel B, Axelrod D, et al. Acute toxicity of high-dose-rate intracavitary brachytherapy with the MammoSite applicator in patients with early-stage breast cancer. Ann Surg Oncol 2004; 11: 739–46
- Shah NM, Tenenholz T, Arthur D, DiPetrillo T, Bornstein B, Cardarelli G, et al. MammoSite and interstitial brachytherapy for accelerated partial breast irradiation: Factors that affect toxicity and cosmesis. Cancer 2004; 101: 727–34
- Dickler A, Kirk MC, Choo J, His WC, Chu J, Dowlatshahi K, et al. Cosmetic outcome and incidence of infection with the MammoSite breast brachytherapy applicator. Breast J 2005; 11: 306–10
- DiFronzo LA, Tsai PI, Hwang JM, Lee JJ, Ryoo MC, Rahimian J, et al. Breast conserving surgery and accelerated partial breast irradiation using the MammoSite system: Initial clinical experience. Arch Surg 2005; 140: 787–94
- Harper JL, Jenrette JM, Vanek KN, Aguero EG, Gillanders WE. Acute complications of MammoSite brachytherapy: A single institution's initial clinical experience. Int J Radiat Oncol Biol Phys 2005; 61: 169–74
- Vicini FA, Beitsch PD, Quiet CA, Keleher A, Garcia D, Snider HC, et al. First analysis of patient demographics, technical reproducibility, cosmesis, and early toxicity: Results of the American Society of Breast Surgeons MammoSite breast brachytherapy trial. Cancer 2005; 104: 1138–48
- Benitez PR, Streeter O, Vicini F, Mehta V, Quiet C, Kuske R, et al. Preliminary results and evaluation of MammoSite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ: A phase II clinical study. Am J Surg 2006; 192: 427–33
- Jeruss, JS, Vicini, FA, Beitsch, DD, Haffty, BG, Quiet, CA, Zannis, VJ, , et al. Initial outcomes for patients treated on the American Society of Breast Surgeons MammoSite clinical trial for ductal carcinoma-in-situ of the breast. Ann Surg Oncol 2006;13:967–76. Epub 2006 May 16.
- Sadeghi A, Prestidge B, Lee JM, Rosenthal A. Evaluation of the surface radiation dose and dose gradient in early stage breast cancer using high-dose-rate brachytherapy MammoSite applicator. Brachytherapy 2006; 5: 230–4
- Chao, KK, Vicini, FA, Wallace, M, Mitchell, C, Chen, P, Ghilezan, M, , et al. Analysis of treatment efficacy, cosmesis, and toxicity using the MammoSite breast brachytherapy catheter to deliver accelerated partial-breast irradiation: The William Beaumont Hospital experience. Int J Radiat Oncol Biol Phys 2007;69:32–40. Epub 2007 Apr 30.
- Chen S, Dickler A, Kirk M, Shah A, Jokich P, Solmos G, et al. Patterns of failure after MammoSite brachytherapy partial breast irradiation: A detailed analysis. Int J Radiat Oncol Biol Phys 2007; 69: 25–31
- Benitez PR, Keisch ME, Vicini F, Stolier A, Scroggins T, Walker A, et al. Five-year results: The initial clinical trial of MammoSite balloon brachytherapy for partial breast irradiation in early-stage breast cancer. Am J Surg 2007; 194: 456–62
- Agawal, A, Beriwal, S, Heron, D, Falk, J, Johnson, R, Mogus, BM, , et al. Accelerated partial breast irradiation: Single institutional experience of 100 patients using MammoSite brachytherapy. Int J Radiat Oncol Biol Phys 2005;63((Suppl 1))s6–s7.
- Evans, SB, Kaufman, SA, Price, LL, Cardarelli, G, DiPetrillo, TA, Wazer, DE, , et al. Persistent seroma after intraoperative placement of MammoSite for accelerated partial breast irradiation: Incidence, pathologic anatomy, and contributing factors. Int J Radiat Oncol Biol Phys 2006;65:333–9. Epub 2006 Mar 20.