To the Editor
The synchronous occurrence of multiple primary dissimilar neoplasms in the general population is rare. Here, we report a rare jejunal gastrointestinal stromal tumor (GIST), an intrahepatic cholangiocarcinoma (ICC), and a pulmonary hamartoma which were all detected simultaneously.
In November 2007, a 56-year-old man was referred to our department because of vague right upper abdominal pain for about one month. No other gastrointestinal symptoms were observed. The patient had a nine year history of hepatitis B virus infection. Ultrasonography of the liver revealed a mass in the right lobe of liver. A physical examination showed no remarkable findings, and hematological and biochemical data were within normal limits; specifically, serum albumin, platelet and WBC counts were within normal limits, and there was no evidence of portal hypertension manifested by esophageal or other varices by scans. The case was positive (defined as > 5 ng/ml; 9 ng/ml in this case) for serum carcinoembryonic antigen (CEA), but negative for serum α-fetoprotein, and carbohydrate antigen 19-9 (CA19-9). This case was also positive for hepatitis B surface antigen, but negative for hepatitis C virus antibody (anti-HCV). The fecal occult blood test result was positive. Fiber colonoscopy revealed no gross abnormalities. [18F]Fluorodeoxyglucose (FDG) positron emission tomography (PET) and computed tomography (CT) scans of the whole body demonstrated three lesions in this patient. One was found in the right liver, another in the right abdominal cavity, and the third in the lower lobe of the right lung. The former two lesions showed a marked increased glucose uptake on FDG-PET, whereas the pulmonary lesion showed no appreciable uptake (). The standardized uptake values of hepatic and abdominal lesions were 10.1 and 7.2, respectively. The pre-operative diagnosis could not be definitely made, and an exploratory laparotomy was performed.
Figure 1. From left to right, Computed tomography (CT), positron emission tomography using [18F]fluorodeoxyglucose (FDG-PET), and fused FDG-PET/CT transaxial imaging of hepatic (A), jejunal (B) and pulmonary (C) lesions. A: CT shows a relatively low-density right intrahepatic mass, with markedly increased glucose uptake on FDG-PET. B: CT shows a median-density right abdominal mass, with markedly increased glucose uptake on FDG-PET. C: CT shows a median-density right pulmonary nodule, with no appreciable glucose uptake on FDG-PET.
![Figure 1. From left to right, Computed tomography (CT), positron emission tomography using [18F]fluorodeoxyglucose (FDG-PET), and fused FDG-PET/CT transaxial imaging of hepatic (A), jejunal (B) and pulmonary (C) lesions. A: CT shows a relatively low-density right intrahepatic mass, with markedly increased glucose uptake on FDG-PET. B: CT shows a median-density right abdominal mass, with markedly increased glucose uptake on FDG-PET. C: CT shows a median-density right pulmonary nodule, with no appreciable glucose uptake on FDG-PET.](/cms/asset/081f7bd2-b9d6-4dd3-98d7-ec2de3b5a14e/ionc_a_376072_f0001_b.jpg)
During the abdominal exploration, two lesions were found. One lesion (measured 5.0×3.0×2.0 cm) was attached to the jejunum wall (20 cm distal to the ligament of Treitz) projecting into the abdominal cavity (A), and another (measured 4.5×4.0×3.5 cm) was located in Couinaund's segment eight of the liver. No other masses were observed inside the abdominopelvic cavity and there was no evidence indicating lymph nodes involvement. The liver appeared cirrhotic. A partial enterectomy, and right partial hepatectomy were performed to radically remove the two lesions inside the abdominal cavity. The proximal and distal resection margins from the jejunal tumor were 5 cm, and the resection margins from the liver tumor were 1 cm. After removal of the two lesions inside the abdomen, an 8 cm dissection in the right diaphragm was made to enter the right thoracic cavity. The right pulmonary tumor (measured 1.0×1.0×0.8 cm) was located on the surface of the inferior border (basal segment) of the lower lobe of lung, and a wedge resection was performed to remove the pulmonary tumor. After that, the dissected right diaphragm was closed with sutures, and a drainage tube was placed in the right subphrenic recess which was removed 3 days postoperatively. The patient tolerated the procedure well, had an uneventful postoperative course, and was discharged on the 11th postoperative day.
Figure 2. The lesion located in the right abdominal cavity attached to the jejunum wall, growing in an extraluminal manner (A). Microscopically, the tumor was composed of fascicles of spindle cells in an edematous background (hematoxylin and eosin stained, ×200) (B), which were positive for CD117 (diaminobenzidine reaction, hematoxylin counterstain, ×200) (C).
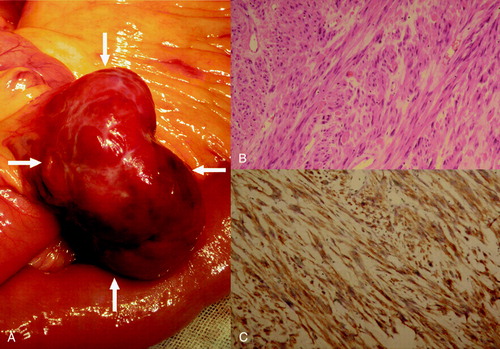
Pathologically, the jejunal lesion showed a well-demarcated tan-colored soft mass arising from the muscular layer and protruding to the serosa with foci of hemorrhage on cut sections. Small ulcer foci could be identified on the jejunal mucosa. Microscopically, the tumor was composed of fascicles of spindle cells in an edematous background. Tumor cells showed moderate atypia with dispersed chromatin and inconspicuous nucleoli. Mitotic figures were seen in about 2 per 50 cells per high power field (B). Immunohistochemical analysis showed a strong expression of vimentin, CD117, and CD34 (C). The proliferative index (Ki-67) was less than 1%. Stains for CK, desmin, and S-100 were negative. Pathologically, a diagnosis of low grade GIST of jejunum was made. The hepatic lesion was pathologically diagnosed as a poorly differentiated intrahepatic cholangiocarcinoma. Resection margins were negative, and there was no evidence of microscopic vascular invasion. The para-tumorous hepatic parenchyma revealed incipient micronodular cirrhosis histologically. As to the pulmonary lesion, a lung hamartoma was pathologically proved.
The patient has been treated with 400 mg imatinib mesylate/day from operative day 8 up to the writing of this report. Twelve months after surgery, the patient repeated PET/CT scans, and had no evidence of tumor recurrence or metastasis.
Discussion
In the case described herein, the GIST was incidentally detected by the whole body PET/CT scan, and occult bleeding may have been the only symptom. Surgical resection of the localized GIST is standard therapy, and the currently accepted procedure is complete resection of the tumor with grossly negative margins Citation[1]. However, optimal management of GIST requires a multidisciplinary approach. Surgery is usually the treatment of choice to obtain immediate symptom relief. Imatinib, an inhibitor of c-kit tyrosine kinase activity, is now the standard post-operative therapy for patients with advanced GIST. The most recent guidelines of the National Comprehensive Cancer Network Citation[2] have recommended the use of imatinib for at least 12 months as adjuvant therapy after the complete resection of the primary GIST.
Synchronous or metachronous incidence of GIST and primary hepatic neoplasms is exceptionally rare. To our knowledge, this is the first case of a diagnosis of a GIST and ICC found to be present synchronously. For this patient, right upper abdominal pain was the only symptom of the ICC. An elevated CEA concentration is generally found with ICC, although it is nonspecific. In this case, the prognosis of coexisting GIST and cholangiocarcinoma is dominated by natural history of the malignant process. In the absence of very efficacious medical agents for cholangiocarcinoma, the outcome will depend very strongly on the effectiveness of surgical removal. In the present case, the absence of lymph node involvement, negative resection margins, a solitary intrahepatic lesion, and lack of microscopic vascular invasion may have contributed to the positive outcome Citation[3], Citation[4].
GIST and cholangiocarcinoma usually carry poor prognoses even after resection. However, this is frequently because the tumors are not found until late, when metastasis has already occurred. While GIST tumors frequently respond to imitinib, cholangiocarcinoma does not. The fact that the patient described here had a 12-month disease-free interval after surgery is likely due to the early, incidental finding of the tumors and prompt removal. The first imaging test conducted in this patient was ultrasonography, which revealed only an intrahepatic lesion. However, a subsequent PET/CT scan of the whole body revealed another two incidental foci, which was confirmed by the postoperative pathologic results (i.e., lesions with a high glucose uptake were pathologically malignant: GIST and ICC). In summary, the whole-body PET/CT scan played an important role in guiding further investigation and enhancing an accurate and early diagnosis for this patient.
Pulmonary hamartomas are benign neoplasms that usually present in asymptomatic adult men. Because they often present as a single peripheral nodular lesions radiographically Citation[5], pulmonary hamartomas can be misinterpreted clinically as metastatic lesions, although PET/CT will show no increased FDG uptake. Wedge resection is adequate for these tumors which have little or no risk of malignant transformation or recurrence.
In summary, we have described the first case of a GIST with a simultaneously diagnosed ICC and pulmonary hamartoma. The whole body PET/CT scan can make possible accurate and early detection of these neoplasms, and appropriate treatment strategy (removal of all these tumors and adjuvant imatinib therapy for the GIST) may contribute to a favorable outcome.
Acknowledgements
The authors thank George Y. Wu, M.D., Ph.D., for his critical review of the manuscript.
References
- Heinrich MC, Corless CL. Gastric GI stromal tumors (GISTs): the role of surgery in the era of targeted therapy. J Surg Oncol 2005; 90: 195–207
- NCCN. Clinical Practice Guidelines in Oncology™ (Soft Tissue Sarcoma). Available at:, , http://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf. Accessed December 13, 2008.
- Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996; 224: 463–73
- Malhi H, Gores GJ. Cholangiocarcinoma: Modern advances in understanding a deadly old disease. J Hepatol 2006; 45: 856–67
- Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc 1996; 71: 14–20