Abstract
Background and purpose: Palliative pelvic radiotherapy (PPRT) is used to treat locally advanced rectal cancer (RC) although symptomatic effects and toxicities are poorly documented. Aims were to evaluate symptom severity, quality of life (QOL) and toxicity after PPRT.
Material and methods: Fifty-one patients with symptomatic primary or recurrent RC prescribed PPRT with fractions of 3 Gy to 30–39 Gy were included. Primary outcome was severity of target symptoms (TS) 12 weeks after PPRT. Pelvic symptom burden, toxicity, and QOL were assessed. Response was defined as patient-reported TS improvement or resolution.
Results: Pain (n = 24), rectal dysfunction (n = 16), and hematochezia (n = 9) were the most common TSs. Overall response rate among evaluable patients 12 weeks after PPRT was 28/33 (85%). Eighteen patients did not complete the study follow-up, 16 due to deteriorating health. TS responses were 10/13 (77%) for pain, 9/10 (90%) for rectal dysfunction, and 8/8 for hematochezia. Non-target pelvic symptom severity decreased and median QOL scores remained stable. There was no grade 4 toxicity. Median survival was nine months.
Conclusions: In the majority of patients with symptomatic primary or recurrent RC, PPRT with 30–39 Gy contributes to pelvic symptom relief, with little toxicity. Patients prescribed PPRT of RC have limited life expectancy. Future studies should investigate simplification of PPRT.
During recent decades, advances in surgical technique, diagnostics, radiotherapy and new oncologic drugs have contributed to improved outcomes in rectal cancer (RC), including prolonged survival in metastatic disease [Citation1]. However, patients with locally recurrent or primarily inoperable RC may still experience a growing pelvic tumor, potentially leading to symptoms, such as pain, obstruction, and hematochezia [Citation2].
Radiotherapy of RC is most often delivered preoperatively with curative intent, either as conventional long-course chemoradiotherapy [Citation3] or as short-course radiotherapy [Citation4], and duration of treatment ranges from 5 to 27 days. Tumor shrinkage in response to radiotherapy is often not apparent radiologically until several weeks after treatment completion [Citation5]. The radiation dose and degree of tumor response required to palliate different pelvic symptoms is unknown, but a recent systematic review of palliative pelvic radiotherapy (PPRT) of RC found symptomatic improvement across a wide range of treatment schedules [Citation2]. Conventional curative fractionation regimens intended to downstage tumors may not be appropriate in the context of symptom palliation, and there is a need for studies evaluating the effects of palliative radiotherapy [Citation6].
Studies of PPRT of RC to date are largely outdated and retrospective, and their validity for today’s clinical decision making is limited [Citation2]. Questions of optimal application of PPRT with regard to indication, dose and timing within the greater context of palliative oncologic treatment, remain unanswered [Citation2]. Randomized studies of palliative radiotherapy in other scenarios have shown that hypofractionated treatment can palliate as effectively as conventional treatment, without increased toxicity [Citation6]. However, differences in cancer biology, constellations of symptoms produced by different cancers, and disparate therapeutic options, preclude direct extrapolation of these results to patients with RC.
Prospective multicenter study of patients undergoing palliative radiotherapy is challenging [Citation7]. After feasibility testing [Citation8], we performed two parallel nationwide prospective multicenter phase II single arm studies (PallRad1) of PPRT in the range of 30–39 Gy for prostate and RCs [Citation9]. The range of doses was chosen because it covered the most commonly used fractionation schedules in Norway when the study was planned.
The primary aim of the current study was to prospectively evaluate the palliative effect of PPRT in patients with a symptomatic primary or recurrent RC 12 weeks after treatment. In addition, we explored quality of life (QOL), symptom status and toxicity at the end of radiotherapy, and six and 12 weeks after radiotherapy.
Methods
Study design and patients
Eight of the nine radiotherapy centers in Norway participated in this phase II study that ran from November 2009 to July 2015.
Eligible patients with RC presented with a symptomatic soft-tissue pelvic mass (primary tumor or recurrence of adenocarcinoma of the rectum), independent of the simultaneous presence of metastases. The study did not standardize staging procedures. Patients were eligible throughout the course of their disease, meaning that they could have received previous treatments for RC (with the exception of previous pelvic radiotherapy). They had to be ≥18 years, with a life expectancy greater than three months. Radiotherapy had to have been prescribed in the range of 30–39 Gy in 3 Gy fractions prior to referral to the study. Patients were ineligible if they were unable to comply with study questionnaires, had started systemic antineoplastic treatment within four weeks prior to baseline, or if this was planned within six weeks after radiotherapy. Patients who had a synchronous pelvic non-RC or other cancer requiring treatment were ineligible, as were those receiving treatment with an investigational drug.
Treatment
When the current study was planned (2009), an informal survey of radiation oncologists in Norway revealed that although several fractionation regimens were used, the most frequent for PPRT of RC were in the range of 3 Gy ×10–13 (unpublished results). In order to limit heterogeneity, the current study was limited to patients scheduled for external beam radiotherapy delivered in 10–13 fractions of 3 Gy. This prescription had to have been made prior to study entry (see inclusion criteria above). Treatment planning was performed by computerized tomography (CT). Gross tumor volume (GTV) encompassed the pelvic tumor, pathologically enlarged lymph nodes, or a combination of these. Planning target volume included the GTV and a margin of 1.0–2.0 cm. Field number and set-up was at the discretion of the treating physician. There were no limitations on other supportive measures including interventions and analgesics during the study.
Data collection
Four study visits were scheduled; at baseline (14–0 days prior to radiotherapy), at the completion of radiotherapy (±3 days), and six and 12 weeks (±7 days) after completion of treatment. Background data pertaining to RC history were collected from patient records. Ancillary palliative procedures and medication use were documented prospectively. Survival data were obtained from the Norwegian Cause of Death Registry.
Symptom and toxicity assessment
Patients were asked at baseline to identify a ‘target symptom’, the chief pelvic complaint that they hoped the radiotherapy would relieve. At each of the three follow-up visits they were asked to describe the TS severity compared to baseline as ‘worse’, ‘unchanged’, ‘improved’ or ‘resolved’ by choosing one of these four anchor-based descriptors. TS response to radiotherapy was defined as either ‘improved’ or ‘resolved’.
To assess QOL and characterize pain the validated Norwegian versions of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30, version 3.0) [Citation10, Citation11] and Brief Pain Inventory short form with body map (BPI) [Citation12, Citation13] were used at each study visit. Questionnaires were self-administered and collected at the radiotherapy centers. Radiotherapists ensured that forms were completed and were available to assist the study participants, as needed. In instances where patients were prevented from attending study follow-up visits, an attempt was made to contact them by telephone and administer the questionnaires via post.
Physicians prospectively graded pre-specified pelvic symptoms and potential toxicities according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE v.3.0) criteria at each study visit [Citation14].
Statistical considerations
The primary endpoint was the proportion of patients reporting improved or resolved TS severity (defined as TS response) compared to baseline at the 12-week follow-up visit. Secondary endpoints were changes in TS severity at the end of treatment and at the six-week follow-up visit, as well as QOL and degree of toxicity at all of the follow-up study visits.
A TS response rate of at least 30–40% was deemed necessary in order to justify subjecting patients to 2–3 weeks of radiotherapy. If the true response rate is 40%, a total of 47 patients would be needed to obtain 90% power to exclude a response rate of <20%, with significance level of 5%. Correspondingly, the power would be 80% with a total of 35 patients. With 40 evaluable patients the maximum length of a 95% confidence interval (CI) for the proportion of responders is ±15%.
With regard to the secondary endpoint, a change of ≥10 points in the EORTC QLQ-C30 global QOL score was considered clinically significant [Citation15]. Assuming a standard deviation in the range of 20–25 [Citation16], 32–51 patients would give a power of 80%. Thus, a total of 40 evaluable patients was deemed sufficient to detect relevant effects on both primary and secondary outcomes.
Descriptive statistics were generated to describe the population, treatment given, and the primary endpoint. 95% CIs were also estimated. Results for the main TS subgroups (pain, hematochezia, and rectal dysfunction) are presented separately due to clinical relevance.
Fisher’s exact test was used to assess the association between selected variables (age >/≤ the median, Eastern Cooperative Oncology Group performance status (ECOG-PS) 0–1 versus 2–4, normal versus low albumin levels, presence or absence of metastases, and whether or not patients had been given prior anti-cancer treatment) and study attrition. Multivariable logistic regression analysis was performed using study attrition as the dependent variable. Likelihood ratio tests were used to compare different models.
Differences in median QOL score from baseline to each follow-up visit were assessed by the two-tailed Wilcoxon-signed rank test (significance level of p < 0.05) for paired data. Toxicity is presented in percent of patients with each grade of symptoms at the four study visits.
In order to describe the study population, Kaplan-Meier survival analysis was performed with the observation time spanning from the start of radiotherapy to death or through 1 October 2015.
Ethical considerations
Study participants gave written informed consent. The study was approved by the Regional Ethical Committee (ref. S-09080c 2009/1695) and the Privacy Protection Council in Norway (ref. 20940) and by hospital institutional boards. The study was registered on ClinicalTrials.gov (ref. NCT01023529).
Results
Patient and treatment characteristics
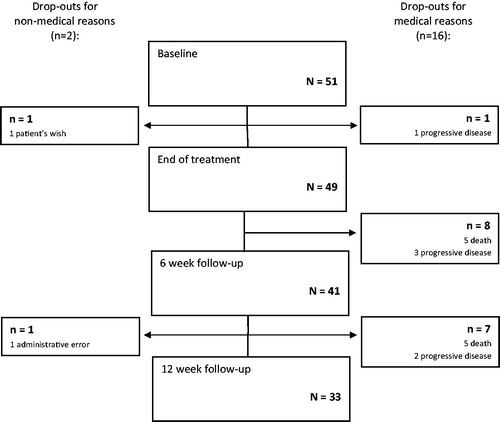
Fifty-one patients were included in the study (). Ten patients died of RC during the study period and six were unable to comply with study procedures due to progressive disease. Two patients dropped out of the study for non-medical reasons. Thirty-three patients (65%) were evaluable at the 12-week follow-up visit.
The median age of included patients was 79 years (). Twelve patients (24%) had recurrent pelvic tumors and 41 (80%) had metastatic disease outside the pelvis. Thirty-two patients (63%) had not received any previous oncologic treatment of their recurrent or metastatic disease and 23 patients (45%) had a colostomy or ileostomy at baseline. Twenty-one patients (41%) used opioids at baseline. The most frequent patient-reported TSs were pain (n = 24; 47%), rectal dysfunction (including obstruction, incontinence, diarrhea, and mucous production) (n = 16; 31%), and hematochezia (n = 9; 18%).
Table 1. Patient characteristics at baseline.
Two variables were identified as independent predictors of study discontinuation due to failing health: albumin <36 g/l [OR 1.31 (95% CI 1.11–1.54)] and age ≤ the median of 79 years [OR 4.79 (95% CI 0.97–23.65)].
PPRT was delivered in 3 Gy fractions to a median total dose of 36 Gy (range 6–39 Gy) (). Five patients had their prescribed radiotherapy altered due to declining health. One of these sustained a hip fracture, three had rapidly deteriorating functional status, and one developed grade 2 nausea and vomiting, prohibiting further radiotherapy.
Table 2. Palliative pelvic radiotherapy.
Symptom palliation
Of the 33 patients evaluable for TS severity 12 weeks after PPRT, 17 (52%) reported complete resolution, 11 (33%) reported improvement, four (12%) remained unchanged and one reported worsening TS severity (, ). Overall, 60% of evaluable patients (28/47) had TS response (improvement or complete resolution) at the end of the radiotherapy course (±3 days) and 85% reported response at the six-week (35/41) and at the 12-week (28/33) follow-ups. Response rates over time differed for each of the TSs (). Eighty-two percent (42/51) of all included patients reported complete resolution or improvement of TS severity at least one study visit.
Figure 2. Patient-reported target symptom severity over time compared with baseline for the three major target symptoms.
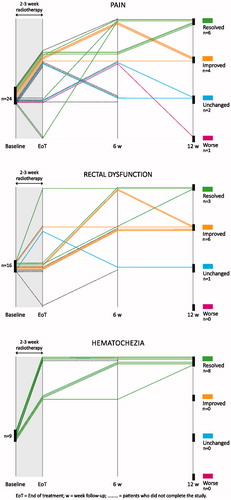
Figure 3. Toxicity. Percent of patients reporting various symptoms at each of the four study visits [B = baseline (n = 51); E = end of treatment (n = 47); 6 = 6-week follow-up (n = 38); 12 = 12-week follow-up (n = 28)]. Symptoms graded according to NCI-CTCAE 3.0. There were no grade 4 symptoms reported.
![Figure 3. Toxicity. Percent of patients reporting various symptoms at each of the four study visits [B = baseline (n = 51); E = end of treatment (n = 47); 6 = 6-week follow-up (n = 38); 12 = 12-week follow-up (n = 28)]. Symptoms graded according to NCI-CTCAE 3.0. There were no grade 4 symptoms reported.](/cms/asset/b6541c4d-dbd3-4664-b4d3-2eda46d8767e/ionc_a_1191666_f0003_c.jpg)
Table 3. Target symptom response and QOL compared to baseline.
According to statistical tests QOL among patients capable of complying with study procedures remained stable throughout the study (), while 38–40% of patients reported clinically significant improvement in QOL at each of the three follow-up visits.
At baseline, 69% (35/51) of patients had ECOG-PS 0-1. This proportion decreased to 57% (29/51) at the end of radiotherapy, 37% (19/51) six weeks after treatment, and 35% (18/51) at the end of the study.
Ancillary palliative procedures
Four patients were also given palliative radiotherapy of other targets (three skeletal, one whole brain) and seven patients started palliative chemotherapy between the six and 12-week study follow-up visits. One patient required a colostomy due to a colonic perforation between the six and 12-week follow-up visits.
Toxicity
There were no grade four toxicities reported, although radiotherapy was terminated after only two fractions (6 Gy) in a patient who developed grade 2 nausea and vomiting. The frequencies of pelvic toxicities, which were primarily proctitis, diarrhea, nausea, dysuria and urinary frequency, all peaked (largely grade 1–2) at the end of PPRT and with the exception of dysuria, these all subsequently decreased to prevalences lower than their baseline levels ().
Survival
At the time of analysis, median duration of follow-up was 31 months (range 5–69), and 43 patients had died (41 due to RC and two due to infections). Median overall survival among the included patients was nine months (range 0–51) from the time of radiotherapy start.
Discussion
This is the first study to prospectively document symptomatic effects and toxicities of PPRT for the treatment of primary and recurrent RC using patient-reported outcomes and active capture of toxicity.
The overall symptomatic results were excellent and best in cases of hematochezia where they were also consistently rapid and durable, at least during the study period. Response rates were good for pain and rectal dysfunction as well although symptomatic improvement was slower and time courses more variable than for hematochezia. This is consistent with findings in other pelvic cancers, such as prostate [Citation9], bladder [Citation17] and gynecological cancers [Citation18], and may reflect the fact that palliation of pain and rectal dysfunction requires a degree of tumor shrinkage.
PPRT in this study was well tolerated, with primarily transient low-grade toxicities reported. Nevertheless, toxicities are likely overestimated due to overlap between the non-TSs caused by the pelvic tumor and pelvic toxicity resulting from radiotherapy. Active capture of expected toxicity after PPRT demonstrated that the multitude of pelvic symptoms present prior to radiotherapy (see baseline levels in ) improved during the course of the study. This clinically significant finding, indicating that PPRT impacts positively on a constellation of simultaneous pelvic symptoms, gives added value to PPRT compared to other modalities which typically target only one or a few isolated symptoms. As with other pelvic tumors, results demonstrate that the positive effects of PPRT outweigh the negative [Citation9].
The group of patients that completed the study benefitted from PPRT in terms of sustained QOL although statistically significant improvement could not be demonstrated, possibly due to the limited number of evaluable patients. On the one hand, this is not surprising as they were most often successfully palliated for the pelvic symptoms which dominated their clinical pictures at baseline. However, the majority of study patients also had distant metastases that largely remained untreated during the 3–4-month study period and several had deteriorating performance status. For the group of patients that completed the study, the benefit of pelvic symptom palliation may have compensated for the burden of progressive metastatic disease. This, however, cannot be generalized to patients with shorter survival times.
The study did not meet its target of 40 evaluable patients despite including 51. Although the intention was to select patients with life expectancies of at least three months, prognostication was, as is often the case, suboptimal [Citation19]. Many patients were given PPRT with 30–39 Gy either because they were unfit for chemotherapy or because they had exhausted their systemic treatment options. The fact that so many patients deteriorated significantly or died during the 3–4-month study period is an important finding in itself and highlights a well established difficulty in conducting research on patients receiving palliative interventions [Citation20]. The limited survival observed in this population brings to question how better to select patients who are clinically stable enough to benefit from two to three weeks of radiotherapy, and whether it is appropriate to use such ‘traditional’ longer course palliative radiotherapy regimens in this population.
While low baseline albumin level was an expected risk factor [Citation21], it was surprising that younger age was also associated with study discontinuation due to deteriorating health. Somewhat unexpectedly, ECOG-PS, the presence of metastatic disease and chemotherapy prior to study entry were not significant predictors of study discontinuation. Younger patients were more likely than older patients to have been pretreated with chemotherapy and although not demonstrated in this small study, one could hypothesize that younger patients were more likely to have been prescribed PPRT at later stages of their disease because palliative systemic treatment was prioritized in earlier phases and PPRT delivered only when patients progressed on chemotherapy [Citation22].
The combination of positive symptomatic effects and modest toxicities shown in this study indicate that radiotherapy with 30–39 Gy in 3 Gy fractions is a good option for many patients. However, the short life expectancies of the patients in this study, coupled with the growing evidence base demonstrating safety and efficacy of short-course radiotherapy (5 Gy × 5) given preoperatively in high-risk RC [Citation23], argues for simpler, shorter treatment schedules in this population. As such, large fractions of 8–10 Gy, repeated as needed, may be appropriate when hematochezia is the TS, whereas 5 Gy × 5 may be preferable if tumor response is sought.
In a study analogous to this one, 40 patients with prostate cancer treated with PPRT in the range of 30–39 Gy, had a 70% overall TS response rate 12 weeks after PPRT, with acceptable toxicity. Hematuria and pain were particularly well palliated, with 11/12 and 7/9 responders, respectively [Citation9]. A randomized study of PPRT of bladder cancer comparing 35 Gy in 10 fractions to 21 Gy in 3 fractions, found no differences in efficacy or toxicity between the two arms (overall 68% of patients achieving symptomatic improvement) three months after treatment [Citation24]. Among 58 patients with bladder cancer, PPRT delivered in weekly fractions of 6–36 Gy resulted in symptomatic response rates of 94% for hematuria and 100% for bladder-related pain [Citation17]. A systematic review of PPRT of cervical cancer, including largely observational, retrospective studies, indicates that repeated large fractions of up to 10 Gy appear effective and well tolerated for control of bleeding (45–100% response rates), and may also have a positive effect on pelvic pain and vaginal discharge [Citation18].
Among patients with inoperable RC, pelvic symptoms may present and recur at any time during the course of the disease. In the study population, PPRT was the first treatment modality for some patients, while others had been treated with up to several lines of palliative chemotherapy first. Judicious prioritization of therapeutic options is particularly important in cases where a symptomatic rectal tumor requires palliation in the presence of metastatic disease, particularly if there is evidence of aggressive tumor biology. Systemic treatment may then be the preferred treatment modality in order to stop progression of life-threatening visceral metastases. As opposed to longer courses of palliative radiotherapy that steal time away from systemic treatment, simplified radiotherapy has the advantage that it can be more easily integrated in treatment algorithms that include systemic therapy. 5 Gy × 5 fractions to the rectal tumor followed by systemic chemotherapy is a good example of such an integrated approach and has been shown to generate a sustained palliative effect of previously untreated RC in patients with metastatic disease [Citation25].
This study has several strengths, most notably its use of patient-evaluated prospective symptom assessment and the active capture of toxicity. As study inclusion and exclusion criteria were relatively non-restrictive, the study sample is representative of patients with RC in whom 30–39 Gy in 3 Gy fractions is the chosen radiotherapy regimen. Patients were recruited from both university-based and regional radiotherapy centers across Norway. In addition, the radiotherapy procedure was standardized, using modern but relatively simple and widely available technology. Patients in whom tumor-directed treatments were planned within the first six weeks after radiotherapy were excluded from the study, thereby limiting major confounders.
However, the study also has weaknesses. Study procedures and follow-up after radiotherapy were limited in order to balance the burden on study participants with the aims of gathering sufficient quantity and quality of data. As such, three follow-up study visits were set, leaving gaps in the data continuum where patients’ clinical situations are not described. Withholding radiotherapy in order to perform a randomized controlled trial with best supportive care as the control arm was deemed unethical based on the positive overall experience with PPRT of RC to date [Citation2]. Similarly, confounding supportive treatments could not be withheld. As such, it is difficult to decipher the isolated value of delivering PPRT in this setting and results must be interpreted for PPRT given in the greater context of palliative oncologic care.
The rapidly changing landscape of palliative oncologic treatment algorithms for RC will likely make it increasingly difficult to deliver PPRT in a trial where systemic oncologic treatment is put on hold in order to allow for a course of PPRT followed by several months of study follow-up. In light of the findings presented here, 2–3 weeks of PPRT for patients with incurable RC should be limited to only those patients with relatively long expected survival. Studies randomizing between 30–39 Gy and shorter courses would therefore likely have difficulty with accrual. Future studies could take a similar approach to this one, simply documenting the effectiveness of and tolerance for 5 Gy × 5 when given with palliative intent. Alternatively, they could investigate single fractions of 8–10 Gy (with the option of re-treatment as needed) for hemostasis in various pelvic tumors, including RC.
Conclusion
PPRT of RC with 30–39 Gy contributes to effective palliation of a range of pelvic symptoms including rectal dysfunction, pain and hematochezia, with acceptable toxicity. A large proportion of patients prescribed PPRT of RC have very limited life expectancies and clinicians should consider this in selecting patients for different fractionation schemes. Future studies should investigate simplification of PPRT, especially for hematochezia, but also for patients with short life expectancy, which could impact significantly both on the individual treatment burden and on resource allocation.
Funding information
This research was funded by a grant from the South-Eastern Norway Regional Health Authority. Special thanks to study radiotherapists at each center for making the study feasible.
Disclosure statement
There are no potential conflicts of interest.
References
- Guren MG, Korner H, Pfeffer F, Myklebust TA, Eriksen MT, Edna TH, et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993-2010. Acta Oncol 2015;54:1–9.
- Cameron MG, Kersten C, Vistad I, Fossa S, Guren MG. Palliative pelvic radiotherapy of symptomatic incurable rectal cancer - a systematic review. Acta Oncol 2014;53:164–73.
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–33.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638–46.
- Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum 2013;56:921–30.
- Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol 2014;32:2913–9.
- Kaasa S, Hjermstad MJ, Loge JH. Methodological and structural challenges in palliative care research: how have we fared in the last decades? Palliat Med 2006;20:727–34.
- Cameron MG, Kersten C, van Helvoirt R, Rohde G, Fossa SD, Vistad I. Patient reported outcomes of symptoms and quality of life among cancer patients treated with palliative pelvic radiation: a pilot study. BMC Res Notes 2011;4:252.
- Cameron MG, Kersten C, Vistad I, van Helvoirt R, Weyde K, Undseth C, et al. Palliative pelvic radiotherapy for symptomatic incurable prostate cancer - A prospective multicenter study. Radiother Oncol 2015;115:314–20.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S, et al. The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer 1995;31A:2260–3.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38.
- Klepstad P, Loge JH, Borchgrevink PC, Mendoza TR, Cleeland CS, Kaasa S. The Norwegian brief pain inventory questionnaire: translation and validation in cancer pain patients. J Pain Symptom Manage 2002;24:517–25.
- Cancer Therapy Evaluation Program D, NCI, NIH, DHHS. Common Terminology Criteria for Adverse Events, Version 3.0. 2003.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res 1996;5:555–67.
- Kouloulias V, Tolia M, Kolliarakis N, Siatelis A, Kelekis N. Evaluation of acute toxicity and symptoms palliation in a hypofractionated weekly schedule of external radiotherapy for elderly patients with muscular invasive bladder cancer. International Braz J Urol: Official Journal of the Brazilian Society of Urology 2013;39:77–82.
- van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol 2011;98:287–91.
- Tseng YD, Krishnan MS, Sullivan AJ, Jones JA, Chow E, Balboni TA. How radiation oncologists evaluate and incorporate life expectancy estimates into the treatment of palliative cancer patients: a survey-based study. Int J Radiat Oncol Biol Phys 2013;87:471–8.
- Jordhoy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med 1999;13:299–310.
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutrition Journal 2010;9:69.
- Sorbye H. Palliative chemotherapy in elderly patients with metastatic colorectal cancer: do we know how it should be used? Acta Oncol 2012;51:819–21.
- Zhou ZR, Liu SX, Zhang TS, Chen LX, Xia J, Hu ZD, et al. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: a systematic review and meta-analysis. Surg Oncol 2014;23:211–21.
- Duchesne GM, Bolger JJ, Griffiths GO, Trevor Roberts J, Graham JD, Hoskin PJ, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys 2000;47:379–88.
- Tyc-Szczepaniak D, Wyrwicz L, Kepka L, Michalski W, Olszyna-Serementa M, Palucki J, et al. Palliative radiotherapy and chemotherapy instead of surgery in symptomatic rectal cancer with synchronous unresectable metastases: a phase II study. Ann Oncol 2013;24:2829–34.