Abstract
Background: The diagnosis of secondary upper limb lymphedema (LE) is complicated by the lack of an agreed-upon measurement tool and diagnostic threshold. The aim of this study was to determine which of the many commonly used and normatively determined clinical diagnostic thresholds has the best diagnostic accuracy of secondary upper limb LE, when compared to diagnosis by an appropriate reference standard, lymphoscintigraphy.
Material and methods: The arms of women treated for breast cancer with and without a previous diagnosis of LE, as well as healthy controls, were assessed using lymphoscintigraphy, bioimpedance spectroscopy (BIS) and perometry. Dermal backflow score determined from lymphoscintigraphy imaging assessment (reference standard) was compared with diagnosis by both commonly used and normatively determined diagnostic thresholds for volume and circumference measurements as well as BIS.
Results: For those with established dermal backflow, all commonly used and normatively determined diagnostic thresholds accurately identified presence of LE compared with lymphoscintigraphy diagnosis. In participants with mild to moderate changes in dermal backflow, only a normatively determined diagnostic threshold, set at two standard deviations above the norm, for arm circumference and full arm BIS were found to have both high sensitivity (81% and 76%, respectively) and specificity (96% and 93%, respectively). For this group, strong, and clinically useful, positive (23 and 10, respectively) and negative likelihood (0.2 and 0.3) ratios were found for both the circumference and bioimpedance diagnostic thresholds.
Conclusion: For the first time, evidence-based clinical diagnostic thresholds have been established for secondary LE. With mild LE, normatively determined circumference and BIS thresholds are superior to the commonly used thresholds.
The need to standardize the diagnosis of upper limb lymphedema (LE) has been called for since the 1930s [Citation1]. However, there is little agreement on how LE should be diagnosed, largely due to three factors: the wide variety of measurement tools and protocols used [Citation2]; the extensive range of diagnostic thresholds available for each measurement tool [Citation3]; and the lack of a diagnostic gold standard tool and threshold [Citation4].
Many tools may be used to diagnosis LE. Circumferential measurements are common and may be used either as raw scores or converted to a volume measurement of the intervening segment, using a geometric formula [Citation5]. Perometry is an alternative method for determining both circumference and limb volume, which derives measurements from the shadow image created as the limb breaks beams of light [Citation5]. Both of these techniques provide measurements of the total limb volume, which includes fat, muscle and bone. In contrast, bioimpedance spectroscopy (BIS) provides a measure of the volume of fluid specific to early LE, extra-cellular fluid (ECF), of which lymph is a major component [Citation6]. For each measurement method, a range of diagnostic thresholds have been used to determine the presence of LE.
Diagnostic thresholds for upper limb LE have originated from a variety of sources. The most commonly used thresholds include: an absolute 200 ml inter-limb volume difference, a 2-cm inter-limb circumference difference, a 5-cm inter-limb difference of the sum of all circumference measurements or a relative percentage difference, often 10% [Citation2]. Many of these appear to have been chosen for their ease of use [Citation7] and evidence is lacking to determine the origin of others. Recently, normatively determined thresholds were derived for circumference and volume measurements [Citation8]. These thresholds, like those derived for BIS [Citation6], account for limb dominance and have been set at three standard deviations (3SD) above the mean of a control population. However, it has been suggested that the 3SD approach may be overly conservative [Citation8,Citation9]. Furthermore, which of these tools and thresholds best identify LE is unknown, as comparison against a reasonable reference standard has not been undertaken.
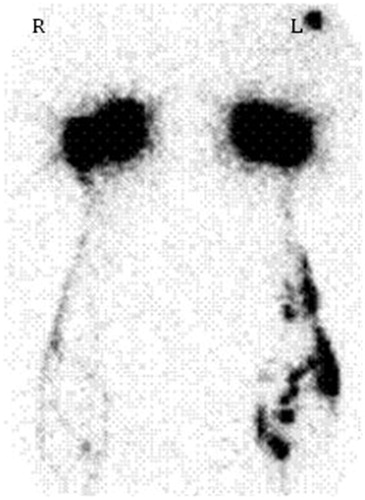
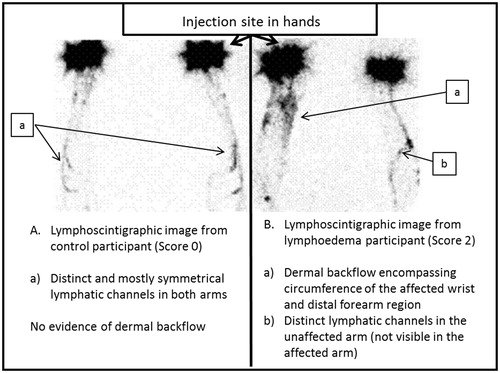
A reference standard requires both a reliable measurement tool and an accepted definitive diagnostic threshold. Lymphoscintigraphy imaging is an ideal reference standard [Citation10] as it specifically examines the anatomy and morphology of the lymphatic system. Until recently, there was not a specific criterion for assessment of upper limb LE by lymphoscintigraphy as most assessments were based on a qualitative assessment of the lymphatic pathways [Citation11]. Dermal backflow (), a feature unique to LE, represents the re-routing of the lymphatic fluid from the obstructed superficial lymphatic system into the sub-cutaneous and dermal space [Citation11]. This change in lymphatic morphology occurs in varying degrees of severity, which can be graded reliably on a four-point scale, [Citation12] thereby providing, for the first time, an imaging-based diagnostic criterion specific to LE.
The aim of this study, therefore, was to determine which normatively determined and/or commonly used diagnostic thresholds are optimal for the diagnosis of LE when compared to diagnosis by lymphoscintigraphy.
Material and methods
Ethical approval
The Human Research Ethics Committees at each institution where the study was conducted gave ethical approval. Participants provided written, informed consent prior to participation.
Participants
Three groups of women were recruited through open advertisements (): 68 women previously treated for LE secondary to unilateral breast cancer (BC) (LE group); 13 without a history of BC or LE (control group); and six women with a history of BC but without an existing diagnosis or signs of LE (BC group). The final group was recruited to confirm that changes seen on lymphoscintigraphy were as a result of the LE and not the BC surgery. The control and BC groups were younger than the LE group (56 and 51 years vs. 61 years; t = −2.11, p = 0.04). On average, the LE group were diagnosed with LE 40 months previously (range 2–142 months), but none had undergone active treatment (e.g. bandaging) within three months of joining the study. The scans of two women with LE and one control participant could not be graded for dermal backflow due to issues with the sub-dermal injections (two) or technologist error (one).
Table 1. Participant characteristics.
Protocol
Lymphoscintigraphy
Women attended a single assessment session. On arrival, the second and fourth web-spaces of both hands were cleaned and a topical anesthetic cream (Emla®) applied to lessen discomfort associated with the injections [Citation13]. Sub-dermal injections of 20–30 MBq 99mTc-antimony colloid (<0.1 ml) were given by an experienced nuclear medicine physician or technologist. Dynamic imaging of the forearm commenced immediately following the injection and lasted for 30 minutes, with the participant seated with their arms placed horizontally on the gamma camera. No hand or arm movement was allowed. Further five-minute dynamic imaging was obtained at 60, 120 and 180 minutes post-injection. Data were collected at one frame/minute using one detector of a dual-detector gamma camera, with a low-energy high resolution collimator and acquired into a 128 × 128 matrix.
A sole experienced nuclear medicine physician, blinded to the previous diagnosis of the patient, completed the qualitative assessment of the lymphoscintigraphy images. The severity of dermal backflow was scored: 0 (no dermal backflow), 1 (a small area or localized pooling); 2 (circumferential dermal backflow pattern present in <50% of the forearm); or 3 (circumferential dermal backflow >50% of the forearm [Citation12]) ().
Clinical measurements
The participants’ arms were measured by BIS and perometry. BIS arm measurements were completed as previously described [Citation14] using an SBF7 impedance spectrometer (Impedimed Ltd, Brisbane, Qld, Australia). All BIS files were processed using software supplied by the manufacturer (Bioimp v.5.2.4.0, Impedimed Ltd.) and the impedance at zero resistance (R0) determined. The inter-limb ratio was determined by comparing the unaffected or non-dominant arm to the affected or dominant arm.
A perometer (Perometer, 1000M Juzo) was used to determine limb circumferences and volumes using a standardized protocol [Citation8]. Bilateral hand lengths (middle finger tip to ulnar styloid) were measured to ensure that limb volume measures commenced from the ulnar styloid [Citation8]. Perometry arm volume measurements (wrist to 40 cm proximally) were determined using the custom-modified Peroplus™ software. In addition, using the perometer software, the circumference of the limb was determined at five locations, starting at the ulnar styloid and then at 10 cm intervals up to 40 cm proximally. For 10 participants (12%) in whom one or both arms were not of sufficient length to enable a 40-cm measurement, the maximum length available bilaterally was used. Arm volume was determined in two ways: (1) by converting the circumference measurements into volumes using the formula for a truncated cone [Citation8]; and (2) using the perometer software. Perometer-determined arm volumes were not available for seven (8%) participants due to software failure. The inter-limb circumference and volume differences were found by subtracting the measurement of the affected side from the unaffected side in the LE and BC groups and the dominant from the non-dominant side for the control group. The sum of the arm circumference (SOAC) measures was calculated for each arm and the inter-limb difference was calculated in the same manner as the circumference and volume differences.
Diagnostic cut-offs
Both normatively determined and commonly used thresholds were examined (). Normatively determined thresholds set at 2SD and 3SD above the mean of a control population were evaluated for arm volume, circumference [Citation8] and BIS [Citation6,Citation14] measurements. As limb dominance significantly affects limb volume and circumference [Citation8] as well as BIS measurements, different thresholds were used depending on dominance of the arm affected or at risk. For the commonly used thresholds, a single threshold was applied regardless of whether the affected arm was dominant or non-dominant, according to the clinical protocol [Citation15].
Table 2. Summary of normatively determined and commonly used diagnostic thresholds examined.
Statistical analysis
The intra-rater reliability of the dermal backflow scoring was assessed for 10 randomly selected files and found to be excellent [ICC(3,1): 0.957; 95% confidence interval (CI) 0.885–0.984]. A one-way ANOVA comparing the inter-limb volume difference and the dermal backflow score was completed to ensure dermal backflow groupings were consistent with inter-limb volume differences. Initially, participants with a dermal backflow score of 0 were categorized as negative for established LE, while those with a score of ≥1 were considered positive for established LE. Pearson’s χ2 was calculated to determine if there was a relationship between the diagnosis by lymphoscintigraphy and each clinical diagnostic threshold. The sensitivity and specificity of each threshold relative to the diagnosis by lymphoscintigraphy was determined.
The next step of the analysis focused on those without severe LE. Participants were excluded if they had clearly established LE (dermal backflow score 3). Data were grouped to compare participants with a dermal backflow score of 1 and 2 (positive for LE) with those with no evidence of dermal backflow (negative for LE; dermal backflow score of 0). Pearson’s χ2 analysis was undertaken, and sensitivity and specificity calculated with 95% CIs. The positive and negative likelihood ratios including 95% CIs were calculated where possible.
Sensitivity and specificity and related analyses were performed using the MedCalc software (version 15.2, Belgium). All other calculations were completed using SPSS for Windows (version 20, IBM, Chicago, IL).
Results
Dermal backflow grading
There was no evidence of dermal backflow on lymphoscintigraphy (Score 0) for ten women in the LE group (14%), nor any of the control and BC participants (). Thirty-eight women (58%) in the LE group had severe dermal backflow (Score 3). The mean inter-limb perometer volume difference increased consistent with the dermal backflow score (F = 88.32, p < 0.001).
Table 3. Dermal backflow score and inter-limb volume difference for each group.
Evaluation of diagnostic criteria
When all participants were included in the analysis, diagnosis of LE for all diagnostic thresholds was excellent compared with diagnosis by lymphoscintigraphy (χ2=25.8–63.8, all significant at p < 0.05; ). The specificity (range 67–94%) and sensitivity (range 92–100%) varied among thresholds.
Table 4. Sensitivity and specificity of normatively determined and commonly used diagnostic thresholds when all participants were included in analysis (dermal backflow score 1–3 vs. 0; n = 84).
When participants with established LE (Score 3) were excluded, fewer diagnostic thresholds could discriminate between those with and without LE compared to lymphoscintigraphy (). Only a single inter-limb circumference difference and a BIS inter-limb ratio, both using a normatively determined 2SD threshold, had high sensitivity, as well as good specificity (over 75%; shaded bars in ). The normatively determined single inter-limb circumference difference [Citation8] had a positive likelihood ratio of 23 (95% CI 3–158) and a negative likelihood ratio of 0.2 (0.1–0.5). The inter-limb whole arm BIS ratio of Cornish [Citation6] had a positive likelihood ratio of 10 (3–40) and a negative likelihood ratio of 0.3 (0.1–0.6).
Table 5. Dermal backflow grade 3 excluded: sensitivity and specificity of diagnostic thresholds (dermal backflow score 1–2 vs. 0; n = 46).
Discussion
This is the first study to establish evidence-based diagnostic criteria for LE against an appropriate reference standard. The two thresholds with the highest sensitivity, as well as good specificity, for detection of mild LE were determined from a normative population, accounting for normal inter-limb variations as well as limb dominance. These findings support the adoption of a more liberal diagnostic threshold set at 2SD above the mean, instead of the previously suggested 3SD threshold [Citation6,Citation8]. For those with mild LE, all of the commonly used and many of the normatively determined diagnostic thresholds had poor sensitivity, increasing the risk that a breast cancer-related lymphedema (BCRL) diagnosis would be missed when these thresholds are used.
Previous attempts to determine appropriate diagnostic thresholds have used questionable reference standards. For example, a prior diagnosis of BCRL has been previously used as a reference standard [Citation16]. The authors noted that some participants did not have clinically evident LE, despite a previous diagnosis. Similarly, we identified ten women with prior diagnoses of LE with no evidence of lymphatic pathway changes on lymphoscintigraphy. These women likely had an alternate explanation for their earlier symptoms, such as transitory treatment-related swelling [Citation17]. As previous diagnosis of LE does not guarantee continued presence of LE, it is not an appropriate reference standard. Other studies have evaluated diagnostic thresholds using measurement tools, such as water displacement or BIS, with non-validated thresholds as the reference standard [Citation18,Citation19]. In contrast, the present study employed lymphoscintigraphy imaging as the reference standard. The presentation of dermal backflow reliably differentiates LE from edemas of other origins [Citation11] and was not present in our control or BC participants.
It should be recognized that the diagnosis of LE may not be definitive, which contributes to difficulties in diagnosing LE. While physical measurements contribute to the diagnosis, other factors, such as change in sensory symptoms and the risk factors for the development of LE, may be used to determine an accurate diagnosis. The likelihood ratios, in concert with a nomogram, allow the results from the BIS or circumference measurement to be added to the clinical picture to provide an indication of whether a person likely has LE [Citation20] (). A single positive or negative test result in the absence or presence of other signs or symptoms, therefore, does not automatically determine that a patient does or does not have the condition. Indeed Cornish and colleagues’ original study on BIS required two measurements at least two weeks apart to be elevated in order to suggest the presence of LE [Citation6]. Nonomograms and likelihood ratios also allow multiple diagnostic methods and thresholds to be used together to improve the likelihood of the correct diagnosis [Citation2]. Future development of a clinical prediction rule will give further guidance to clinicians in determining if LE is present and who would benefit from additional exploration of their diagnosis using imaging techniques, including lymphoscintigraphy.
Figure 3. Example use of a diagnostic nomogram for a patient after sentinel node biopsy. A recent meta-analysis found that a patient who underwent a sentinel node biopsy as a part of their treatment for breast cancer has a 5% chance of developing BCRL [Citation2]. If this is all that was known about the patient, the pretest probability that they have lymphedema is therefore 5%. If a single elevated inter-limb circumference difference was found, with a positive likelihood ratio of 23, the post-test probability of BCRL is 50%. If a positive full arm BIS ratio was then found, which has a positive likelihood ratio of 10, the post-test probability that this patient has BCRL is now over 90%.
![Figure 3. Example use of a diagnostic nomogram for a patient after sentinel node biopsy. A recent meta-analysis found that a patient who underwent a sentinel node biopsy as a part of their treatment for breast cancer has a 5% chance of developing BCRL [Citation2]. If this is all that was known about the patient, the pretest probability that they have lymphedema is therefore 5%. If a single elevated inter-limb circumference difference was found, with a positive likelihood ratio of 23, the post-test probability of BCRL is 50%. If a positive full arm BIS ratio was then found, which has a positive likelihood ratio of 10, the post-test probability that this patient has BCRL is now over 90%.](/cms/asset/59f94bf6-1991-4af2-b999-89dfad0f468a/ionc_a_1191668_f0003_b.jpg)
There are a few issues to consider in relation to these findings. It was initially surprising to find the superiority of diagnosis using circumference measurements over BIS, as BIS is often suggested as the ideal tool for detection of mild LE [Citation21]. On reflection, the higher positive likelihood ratio of the circumference threshold highlights the importance of localized changes in the detection of mild LE; whole arm BIS measurements may not detect localized changes [Citation22]. Furthermore, the sensitivity of BIS is improved where change from baseline, i.e. pre-operative measurements, is used as the criterion [Citation6]. Second, the majority of our participants had extensive dermal backflow (Score 3) and were therefore excluded from analysis to determine the diagnostic abilities of each threshold on those with mild LE. Although the excluded participants had extensive changes to their lymphatic pathways, not all would traditionally have been categorized as having severe LE based on their inter-limb volume difference (range 180–1037 ml), highlighting a weakness of using volume-based thresholds as a reference standard. Third, in determining the presence or absence of LE clinically, we focused solely on the results of physical measurements, such as inter-limb volume, circumference or BIS differences, without consideration of sensory changes or other indicators of LE, such as the presence of pitting or fibrosis. However, diagnosis is frequently determined solely on the results of one physical measurement method [Citation23]. Finally, the present study focused on women living in the community for whom pre-operative measurements were not available. The next step would be to evaluate the utility of these thresholds in a population with pre-operative measurements.
In conclusion, this study addresses an issue first raised over 75 years ago; the need for a standardized approach to upper limb LE diagnosis [Citation1]. When benchmarked against an appropriate reference standard, only two diagnostic criteria, both normatively determined, had suitable sensitivity and specificity to detect mild BCRL. This provides the first evidence-based approach to the diagnosis of LE.
Acknowledgments
The research presented in this study was funded by Cancer Australia and the National Breast Cancer Foundation. ESD was supported by the National Health and Medical Research Council, Australia. SLK is supported by National Breast Cancer Foundation, Australia. Cancer Australia, National Breast Cancer Foundation nor the National Health and Medical Research Council Australia had any role in the preparation of the manuscript.
Disclosure statement
None.
References
- Allen EV. Lymphedema of the extremities - Classification etiology and differential diagnosis - A study of three hundred cases. Arch Int Med 1934 Oct;54:606–24.
- Disipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500–15.
- Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-Related Lymphedema Risk Factors, Diagnosis, Treatment, and Impact: A Review. J Clin Oncol 2012 Oct;30:3726–33.
- Kosir MA, Rymal C, Koppolu P, Hryniuk L, Darga L, Du W, et al. Surgical outcomes after breast cancer surgery: measuring acute lymphedema. J Surg Res 2001 Feb;95:147–51.
- Czerniec SA, Ward LC, Refshauge KM, Beith J, Lee MJ, York S, et al. Assessment of breast cancer-related arm lymphedema-comparison of physical measurement methods and self-report. Cancer Investigation 2010 Jan;28:54–62.
- Cornish BH, Chapman M, Hirst C, Mirolo B, Bunce IH, Ward LC, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 2001 Mar;34:2–11.
- Kissin MW, Dellarovere GQ, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. Br J Surg 1986 Jul;73:580–4.
- Dylke ES, Yee J, Ward LC, Foroughi N, Kilbreath SL. Normative volume difference between the dominant and nondominant upper limbs in healthy older women. Lymphat Res Biol 2012;10:182–8.
- Fu MR, Cleland CM, Guth AA, Kayal M, Haber J, Cartwright F, et al. L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. Lymphology 2013 Jun;46:85–96.
- Lee BB, Laredo J. Contemporary role of lymphoscintigraphy: we can no longer afford to ignore!. Phlebology 2011 Aug;26:177–8.
- Keeley V. The use of lymphoscintigraphy in the management of chronic oedema. J Lymphoedema 2006;1:42–57.
- Dylke ES, McEntee MF, Schembri GP, Brennan PC, Bailey E, Ward LC, et al. Reliability of a radiological grading system for dermal backflow in lymphoscintigraphy imaging. Acad Radiol 2013;20:758–63.
- Dunson GL, Thrall JH, Stevenso J, Pinsky SM. 99mTc minicolloid for radionuclide lymphography. Radiology 1973;109:387–92.
- Ward LC, Dylke E, Czerniec S, Isenring E, Kilbreath SL. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol 2011;9:47–51.
- Armer JM, Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest 2005;23:76–83.
- Smoot BJ, Wong JF, Dodd MJ. Comparison of diagnostic accuracy of clinical measures of breast cancer-related lymphedema: area under the curve. Arch Phys Med Rehabil 2011 Apr;92:603–10.
- Kilbreath SL, Lee M-J, Refshauge KM, Beith JM, Ward LC, Simpson JM, et al. Transient swelling versus lymphoedema in the first year following surgery for breast cancer. Supportive Care in Cancer 2013 Aug;21:2207–15.
- Hayes S, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema secondary to breast cancer: How choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology 2008 Mar;41:18–28.
- Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat 2015 May;151:121–9.
- Akobeng AK. Understanding diagnostic tests 2: likelihood ratios, pre- and post-test probabilities and their use in clinical practice. Acta Paediatr 2006;96:487–91.
- Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol 2006;4:51–6.
- Svensson BJ, Dylke ES, Ward LC, Kilbreath SL. Segmental impedance thresholds for early detection of unilateral Upper Limb Swelling. Lymphat Res Biol 2015;13:253–9.
- Torres Lacomba M, Sanchez MJY, Goni AZ, Merino DP, del Moral OM, Tellez EC, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ 2010 Jan;340:b5396.