Abstract
Background: Decision making regarding cancer treatment is challenging and there is a need for clinical parameters that can guide these decisions. As physical performance appears to be a reflection of health status, the aim of this systematic review is to assess whether physical performance tests (PPTs) are predictive of the clinical outcome and treatment tolerance in cancer patients.
Methods: A literature search was conducted on 2 April 2015 in the electronic databases Medline and Embase to identify studies focusing on the association between objectively measured PPTs and outcome. No limitations in language or publication dates were applied.
Results: The search retrieved 9680 articles, 16 publications were included involving 4187 patients with various cancer types and different treatments. Reported median or mean age varied from 58 to 78 years. Nine studies used the Timed Up & Go (TUG) test, five the Short Physical Performance Battery (SPPB) and five studies focused on gait speed. Poorer TUG, SPPB and gait speed outcome were associated with decreased survival. TUG, SPPB and gait speed were also associated with treatment-related complications. Furthermore, two studies reported an association between poorer TUG and SPPB outcome with higher rates of functional decline.
Conclusion: PPTs appear to show a significant correlation with survival and these tests could be used as a prognostic tool, particular for older adult patients. A less explicit correlation for treatment-related complications and functional decline was also found. To optimize decision making, future research should focus on developing and validating individualized treatment algorithms that incorporate PPTs in addition to cancer- and treatment-related variables.
The incidence of cancer has been rising and is expected to continue to increase even further due to higher life expectancy, aging of the population and active early cancer detection programs [Citation1–7]. Over 45% of newly diagnosed cancer patients are currently older than 70 years of age [Citation8]. Treating vulnerable cancer patients can result in different outcomes compared to their healthy counterparts. The decision-making process is challenging and there is a need for clinical parameters that can guide treatment decisions.
Cancer treatment can result in a decrease in both physical- and role functioning with reported rates of over 60% in post-treatment survivors aged 80 years or older [Citation9]. Post-treatment morbidity is often increased in the older adults and is associated with higher long-term mortality rates [Citation10].
Currently, multidisciplinary decision making regarding oncologic treatment – such as chemotherapy – for patients with cancer are often based on clinical impressions and chronological age [Citation11]. However, various geriatric conditions appear to be of some value in predicting outcome in older adult patients with cancer [Citation12]. One instrument that is often suggested as useful for obtaining a thorough overview of patients’ health by determining the functional, cognitive and psychosocial status in addition to comorbidity, medication use and nutritional status, is the geriatric assessment [Citation13,Citation14]. However, its time-consuming nature renders it difficult to implement in daily practice.
Less elaborate than a full assessment, physical performance appears to be another method for assessing health status, but it is unclear whether it is useful in predicting oncologic treatment outcome. The aim of this systematic review is to assess whether physical performance tests (PPTs) are of predictive value for clinical outcome and treatment tolerance in cancer patients undergoing oncologic therapy by using all relevant evidence.
Methods
Search strategy and selection criteria
A search was performed on 2 April 2015 in the electronic databases Medline and Embase using the following search terms and their synonyms: ‘physical performance’ and ‘cancer’. The details of the search can be found in Appendix 1 (available online at http://www.informahealthcare.com). No limitations in language or publication dates were applied, but the search was limited to studies in adults.
The titles and abstracts of all studies retrieved by the search were assessed by one investigator (NV) to determine which were eligible for further investigation. For this review, a physical performance measure was defined as an objective mobility test which is associated with physical functioning and that can be performed as a quick bedside screening. Tests addressing measurement of muscle-strength or endurance capacity were not included.
In addition, studies were excluded if they did not assess a physical performance measure, or a relevant outcome measure, if they included non-cancer patients, or consisted of abstracts containing insufficient data.
All potentially relevant articles were subsequently screened as full text by two authors (NV and AS). In case only an abstract was available, we attempted to find a final report of the study by searching Embase and Medline using the names of first, second and/or final authors as well as keywords from the title. In case of insufficient data in the original publication, authors were contacted wherever possible to retrieve additional data. Finally, citations of included publications were cross-referenced to retrieve any additional relevant studies.
Data extraction and quality assessment
Two reviewers (NV and AS) independently judged the study design and results for each eligible study. Items that were extracted were study setting and design, study population [number of patients, me(di)an age, sex, type of cancer and treatment], PPTs used and the reported results on the association between the PPTs and the different outcome measures.
The methodological quality of each of the studies was independently assessed by two reviewers (NV and AS), using the Newcastle–Ottawa Scale [Citation15] adapted to this subject (Appendix 2a, available online at http://www.informahealthcare.com). Inter-reviewer disagreement was discussed during a consensus meeting and in case of persisting disagreement, the assistance of a third reviewer (MH) was enlisted.
Data synthesis and analysis
As a result of heterogeneity in study designs, diversity of patient populations and the wide variety in content of the PPTs and the interpretation of the results with various outcome measures, a formal meta-analysis was considered unfeasible. We summarized the study results to describe our main outcomes of interest. Studies performing multivariable analyses were screened for known potential confounders such as age, sex, tumor type, stage and performance status.
Results
Search outcome
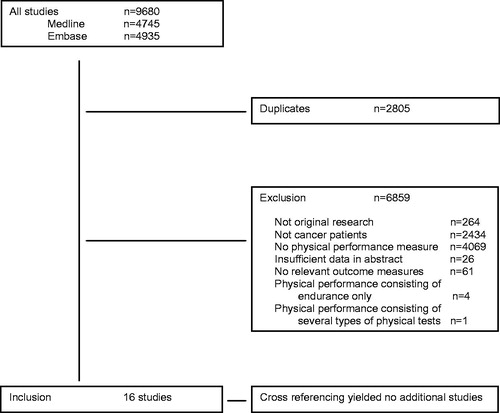
The literature search identified 9680 citations (4745 from Medline and 4935 from Embase), of which 2805 were duplicates and 6859 publications were excluded for other reasons (). Finally, 16 publications from 15 studies [Citation16–31], involving 4187 patients, were included in the analysis. Cross-referencing yielded no additional results.
Study characteristics
An overview of the 16 included papers can be found in . Seven reported on a heterogeneous sample of patients with various cancer types who received various therapies [Citation22–26,Citation28,Citation31]. Four studies focused on patients with hematological malignancies receiving chemotherapy or hematopoietic cell transplantation [Citation20,Citation21,Citation27,Citation29]. Two papers addressed gynecological cancer receiving various treatment types [Citation17,Citation18]. One study included patients with pancreatic tumors undergoing pancreaticoduodenectomy [Citation19], another reported on colorectal cancer patients receiving chemotherapy [Citation30], and finally one analysis included patients with non-small cell lung carcinoma (NSCLC) undergoing chemotherapy [Citation16].
Table 1. Baseline characteristics.
The median number of patients was 202 (range 38–1130) with a reported median or mean age varying from 58 to 78 years.
A total of 13 papers (81%) used one PPT [Citation16,Citation20–31], the other papers (19%) analyzed two tests [Citation17–19]. The Timed Up and Go (TUG) test was used in nine articles [Citation16,Citation20–26,Citation31]. The Short Physical Performance Battery (SPPB) was used in five papers [Citation17–19,Citation27,Citation30]. Gait speed was used in five papers of which four measured 4-m (15 feet) walking time [Citation17–19,Citation29] and one measured 20-m walking time [Citation28]. Details on the methodology of these PPTs can be found in .
Table 2. Overview of the physical performance tests.
Eleven papers reported the association between physical performance and survival [Citation16,Citation18,Citation20–22,Citation24,Citation26–29,Citation31]. The association between physical performance and complications (including chemotherapy toxicity) was addressed in five papers [Citation17,Citation19,Citation24,Citation25,Citation30]; three addressed functional decline [Citation19,Citation23,Citation28].
Quality assessment
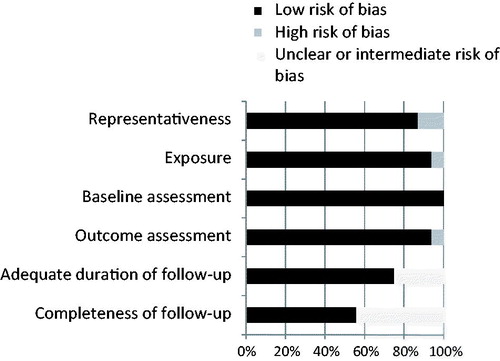
The results of the quality assessment can be found in ; overall, the quality was good. Most studies used representative populations and provided a clear description of the methods used in assessing physical performance in addition to clear outcome definitions and with sufficient follow-up percentages. For the studies performing multivariable analysis, 89% corrected for known prognostic variables such as age, sex, tumor type, stage and performance status (Appendix 3 available online at http://www.informahealthcare.com). However, two publications consisted of conference abstracts containing only limited data.
Physical performance associations
Survival
Each of the physical performance measures was strongly associated with survival: 8 of 11 articles (73%) reported an association in univariate analysis () [Citation16,Citation18,Citation22,Citation26–29,Citation31]. Six reports performed a multivariable analysis, all of which reported a significant association between performance tests and survival [Citation18,Citation21,Citation27–29,Citation31], although in one study this applied only to patients with metastatic disease [Citation28].
Table 3. Associations between physical performance and outcomes.
Five of seven (71%) studies found an univariate association between a prolonged TUG time and survival [Citation16,Citation21,Citation22,Citation26,Citation31]. Of these five, one report found the association to be solely significant after correcting for potential confounders in the multivariable analysis [Citation21]. Another study reported a significant association between TUG time and survival in both the univariate and multivariable analysis [Citation31]. Quantitative estimates of the associations (odds ratios/hazard ratios) can be found in Appendix 4 (available online at http://www.informahealthcare.com).
Complications
Four of the five reports (80%) addressing treatment-related complications found an univariate association with physical performance () [Citation17,Citation24,Citation25,Citation30]. Of these, two out of two studies reported this association for the TUG test [Citation24,Citation25] with one multivariable analysis [Citation24]; the SPPB test was associated in two out of three reports [Citation17,Citation30] within the univariate analysis and finally one out of two reports found gait speed to be significantly associated with treatment-related complications [Citation17] within univariate analysis.
Functional decline
There was a significant association in univariate analysis between physical performance and further functional decline due to treatment in three () [Citation19,Citation23,Citation28]. Of these, one reported the association merely in a non-metastatic subgroup performing the gait speed test [Citation28]. Another study reported an association in the performed SPPB test but did not find it for the gait speed test [Citation19]. The TUG test was studied in one report and it was found to be significantly associated with functional decline in the univariate analysis [Citation23], but not after correcting for potential confounders.
Comparison of physical performance measures
Three studies [Citation17–19] compared two different physical performance measures with each other: gait speed and the SPPB test. One study [Citation17] reported an univariate association between both worse gait speed and SPPB with complications, another study [Citation18] reported univariate and multivariable associations between both tests and survival. The final study [Citation19] did not find an association between gait speed or the SPPB test with complications, but they reported an association within the univariate analysis between worse SPPB outcome and higher rates of functional decline.
Discussion
This review provides an overview of all currently available evidence on the prognostic value of physical performance measures in cancer patients. In the 16 included publications, physical performance appears to be correlated with several outcome measures, particularly survival. Potentially, these tests could therefore be used as a stratification tool in oncologic decision making for, in particular, older patients with cancer.
It is the older adults that suffer most frequently from malignancies, and treatment decisions in these patients can be challenging due to co-existing health issues and functional or cognitive decline. Therefore, developing treatment algorithms for these vulnerable patients might be beneficial. These algorithms could be able to identify patients in which the risk of cancer-related complications and mortality is exceeded by the risk of treatment-related complications and mortality or death due to concurrent conditions. Given the heterogeneity within the older adult population, age itself is not a useful selection tool for oncologic treatment [Citation32] and thus patients should be managed according their individual health status. This can be objectified with current scoring systems such as the ASA-score for surgical patients [Citation33], the Karnofsky Performance Status score [Citation34] and Eastern Cooperative Oncology Group performance status score [Citation35]. However, these scores alone appear to be insufficient for older adult cancer patients, as they do not seem to adequately differentiate within this heterogeneous population [Citation36]. Recent research has focused on using geriatric assessments to provide a more comprehensive overview of an older patients’ overall health status across multiple domains such as physical, psychosocial and functional status [Citation37,Citation38]. Although these assessments yield a wealth of information, their time-consuming nature has hindered a widespread incorporation into daily clinical practice.
Various physical performance measures could present an interesting alternative, as it appears to be a reflection of health status and poor test scores are prognostic for health events [Citation39]. In fact, physical performance could be considered as a summary indicator of vitality because it integrates known and unrecognized disturbances in multiple organ systems such as heart, lungs, circulatory and musculoskeletal systems [Citation40]. In line with our findings, a pooled analysis of nine cohort studies conducted in community dwelling older adults confirmed that physical performance was associated with survival [Citation40]. Another study concluded that a walking speed of slower than 1.36 m/s is associated with poorer overall health status in older community dwelling men and formed a risk factor for mortality [Citation41]. In addition, poor physical performance may result in a vicious cycle of reduced physical activity and deconditioning that has a direct effect on health and survival [Citation40,Citation42]. Furthermore, poor performance prior to treatment also increases the likelihood that a greater part of the survival period will be spent in disabled states [Citation43], which may be a factor to incorporate in treatment decision making.
However, the lack of homogeneity in the studies included in our review and in the study outcomes means that cautious interpretation of the results is warranted. For example, it is too early to assign or withhold a particular cancer treatment based solely on a patient’s PPT score. In future, we believe that more research on the predictive value of physical performance measures should be conducted. Tests which are not only associated with mobility but (also) include other measures of physical performance such as ‘the physical performance test’ [Citation44,Citation45], grip strength test and endurance capacity test, could be of predictive value and should be more investigated in future as well. Developing and validating treatment algorithms that incorporate these instruments, in addition to currently available data regarding the malignancy and treatment, could be used as a stratification tool in decision making in the older cancer patient.
This review has several limitations. First, the included studies were heterogeneous in their patient populations and treatments in addition to the included PPTs and the interpretation of those tests. Across studies, different cutoff values were used for the same instruments. In addition, studies had different follow-up times for survival (ranging from months to several years) and the definitions used for complications (i.e. postoperative morbidity and chemotoxicity) and functional decline differed. As a result, data could not be combined in a formal meta-analysis. Second, most studies only provided univariate results. Therefore, we were unable to establish whether the associations that were found were maintained after correction for possible confounders. Finally, we were unable to definitively exclude a publication bias. However, we believe this risk is limited as most studies did not exclusively focus on physical performance and published negative results for some of the other investigated items. Despite these limitations, this review demonstrates that a relatively simple test might provide highly relevant information regarding the risk of mortality, treatment-related complications and functional decline.
Conclusion
This review demonstrates that PPTs appear to show a significant correlation with survival and these tests could be used as a prognostic tool, particularly for older adult patients. A less explicit correlation for treatment-related complications and functional decline was also found. To optimize decision making for the rapidly growing, heterogeneous group of older cancer patients, future research should focus on developing and validating individualized treatment algorithms that incorporate PPTs in addition to cancer- and treatment-related variables.
Appendix_4.doc
Download MS Word (184.5 KB)Appendix_3.docx
Download MS Word (20.1 KB)Appendix_2b.docx
Download MS Word (28.4 KB)Appendix_2a.docx
Download MS Word (13.6 KB)Appendix_1.docx
Download MS Word (11.6 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This study was supported by the Aart Huisman Scholarship for research in geriatric oncology and the Cornelis Visser Foundation.
References
- Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730–56.
- Active early detection colon cancer programs: “Rijksinstituut voor Volksgezondheid en Milieu”, bevolkingsonderzoek darmkanker [Internet]. [cited 2014 Feb 1]. Available from: www.RIVM.nl
- Jemal A, Bray F, Center MM, et al. Global Cancer Statistics: 2011. CA Cancer J Clin 2011;61:69–90.
- Bretthauer M. Colorectal cancer screening. Journal of Internal Medicine 2011;270:87–98.
- Hewitson P, Glasziou P, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. Cochrane Database Syst Rev 2007;24:CD001216.
- Comparetto C, Borruto F. Cervical cancer screening: a never-ending developing program. World J Clin Cases 2015;3:614–24.
- Bretthauer M, Kalager M. Principles, effectiveness and caveats in screening for cancer. Br J Surg 2013;100:55–65. Vol.
- Netherlands Cancer registry [Internet]. [cited 2015 Jan 1]. Available from: www.cijfersoverkanker.nl
- Hamaker ME, Prins MC, Schiphorst AH, et al. Long-term changes in physical capacity after colorectal cancer treatment. J Geriatr Oncol 2015;6:153–64.
- Verweij NM, Schiphorst A, Pronk A, et al. Conventional and laparoscopic colon resections in the elderly. World J Color Surg 2015;5:3
- Hamaker ME, van Rixtel B, Thunnissen P, et al. Multidisciplinary decision-making on chemotherapy for colorectal cancer: an age-based comparison. J Geriatr Oncol 2015;6:225–32.
- Hamaker ME, Vos AG, Smorenburg CH, et al. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. The Oncologist 2012;17:1439–49.
- Brunello A, Sandri R, Extermann M. Multidimensional geriatric evaluation for older cancer patients as a clinical and research tool. Cancer Treat Rev 2009;35:487–92.
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824–31.
- Wells GA, Shea B, O ’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in metaanalysis. 2014; [Internet]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Biesma B, Wymenga AN, Vincent A, et al. Quality of life, geriatric assessment and survival in elderly patients with non-small-cell lung cancer treated with carboplatin-gemcitabine or carboplatin-paclitaxel: NVALT-3 a phase III study. Ann Oncol 2011;22:1520–7.
- Cerullo F, Coloca G, Ferini A, et al. Misure di funzione fisica in oncogeriatria. G Gerontol 2011;59:265–72.
- Cesari M, Cerullo F, Zamboni V, et al. Functional status and mortality in older women with gynecological cancer. J Gerontol a Biol Sci Med Sci 2013;68:1129–33.
- Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg 2014;259:960–5.
- Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica 2013;98:208–16.
- Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann Hematol 2014;93:1031–40.
- Honecker FU, Wedding U, Rettig K, et al. Use of the Comprehensive Geriatric Assessment (CGA) in elderly patients (pts) with solid tumors to predict mortality. J Clin Oncol 2009;27:9549.
- Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol 2013;31:3877–82.
- Huisman MG, Van Leeuwen BL, Ugolini G, et al. “Timed Up & Go”: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS One 2014;9:e86863.
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.
- Kanesvaran R, Li H, Koo K-N, et al. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly asian patients with cancer. J Clin Oncol 2011;29:3620–7.
- Klepin HD, Geiger AM, Tooze J, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013;121:4287–94.
- Klepin HD, Geiger AM, Tooze J, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82.
- Muffly L, Kocherginsky M, Stock W, et al. Geriatric Assessment (GA) to predict survival in older allogeneic Hematopoietic Cell Transplantation (HCT) recipients. Biol Blood Marrow Transplant 2014;20:S39–S40.
- Ramsdale E, Bylow K, Polite B, et al. Relationship between components of the Comprehensive Geriatric Assessment (CGA), chemotherapy dose intensity and overall survival in a colorectal cancer (CRC) cohort age 65 and over. J Am Geriatr Soc 2012;60:S2.
- Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol 2012;30:1829–34.
- Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy: a systematic review. Leuk Res 2014;38:275–83.
- Hackett NJ, De Oliveira GS, Jain UK, et al. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg 2015;18:184–90.
- Terret C, Albrand G, Moncenix G, et al. Karnofsky Performance Scale (KPS) or Physical Performance Test (PPT)? That is the question. Critic Rev Oncol Hematol 2011;77:142–7.
- Park C-M, Koh Y, Jeon K, et al. Impact of Eastern cooperative oncology group performance status on hospital mortality in critically ill patients. J Crit Care 2014;29:409–13.
- Owusua C, Koroukianb SM, Schluchter M, et al. Screening older cancer patients for a Comprehensive Geriatric Assessment: A comparison of three instruments. J Geriatr Oncol 2011;2:121–9.
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–603.
- Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241–52.
- Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc 2009;57:251–9.
- Studenski S, Perera S, Patel K. Gait speed and survival in older adults. JAMA 2011;305:50–8.
- Stanaway FF, Gnjidic D, Blyth FM, et al. How fast does the Grim Reaper walk? Receiver operating characteristics curve analysis in healthy men aged 70 and over. BMJ 2011;343:d7679.
- Cesari M, Kritchevsky SB, Penninx BWHJ, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2005;53:1675–80.
- Keeler E, Guralnik JM, Tian H, et al. The impact of functional status on life expectancy in older persons. Journals Gerontol - Ser a Biol Sci Med Sci 2010;65 A:727–33.
- Augschoell J, Kemmler G, Hamaker ME, et al. PPT and VES-13 in elderly patients with cancer: Evaluation in multidimensional geriatric assessment and prediction of survival. J Geriatr Oncol 2014;5:415–21.
- Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The physical performance test. J Am Geriatr Soc 1990;38:1105–12.
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8.
- Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–61.