Abstract
Background: Population-based studies for gastric adenocarcinoma are scarce, particularly studies conducted within a defined geographical area with publicly available censuses that allow incidence rates to be calculated.
Material and methods: Population-based study in Central Norway from 2001 to 2011, covering a population of 636 000–680 000, respectively. Patients were identified through the Cancer Registry of Norway and the Norwegian Patient Register, and were characterized by data from individual electronic patient records. Outcomes were compared across the early and the late half of the study period.
Results: A total of 878 patients were identified with a median age of 76.2 years. The male to female ratio was 1.72. Annual world age-standardized incidence was 8.0/105 and 3.6/105, respectively. The Lauren diffuse type was significantly more frequent among patients below 60 years, among females and for non-cardia cancers, compared to their counterparts (p < .001). The Lauren mixed type had a stable proportion of around 13% irrespective of age, sex or tumor location. Early gastric cancers (EGC) represented 8.3% of the cases, whereas 44% of all patients were diagnosed with metastatic disease. In males, the proportion of cardia cancers increased from 29.7% to 39.1% during the study period (p = .005). The five-year overall survival was 16%, and was substantially better for the Lauren intestinal type compared to the diffuse type, log-rank p = .003. The R0-R1 resection rate was 39%, with a corresponding five-year survival of 40.9%.
Conclusions: This study provides population-derived data lacking in hospital-based studies. Lauren categories with epidemiological aspects and clinical outcomes are displayed. Gastric cancer was associated with a dismal prognosis. Few patients had EGC and close to 50% had metastatic disease. Many were too old or frail to be considered for surgery.
Gastric cancer ranks worldwide as the fifth most frequent malignancy. The majority of cases are discovered at advanced stages, at least in Europe and North America, and death tolls remains second only to lung and liver cancer [Citation1]. The incidence has steadily decreased during the last 50 years, but still remains substantially higher in some Eastern countries compared to the West. In Norway, the annual caseload has been mitigated from 1500 in the late 1950s to an annual average of 520 for the period 2005–2009, in spite of a population that increased from 3.5 million to 4.7 million people during the same period. This corresponds to a decline in world age-standardized incidence from 37.2 to 7.0 per 100 000 for males and from 22.0 to 4.0 per 100 000 for females, for the respective periods [Citation2].
The majority of published series on gastric cancer are hospital-based or from randomized controlled trials. They are inherently highly selected, and reported outcomes are influenced accordingly. Population-based studies that include all patients, irrespective of age, disease stage or treatment are few, to some extent incomplete, and seldom report on individual patient data [Citation3–7].
In Norway, legislation demands that clinicians report every case of cancer nominally to the Cancer Registry of Norway (CRN). In addition, pathology departments are obliged to report any biopsy or resection specimen harboring neoplasia to the CRN. This double route of reporting secures a reliable national registration of new onset cases of malignancies. When this legislation appeared in the early 1950s, it was, from a global perspective, pioneering.
A mercantile system termed the Norwegian Patient Register (NPR), launched in 2000, reports on all patients discharged from hospital irrespective of diagnosis. Since 2008, data have been nominally stored. A high degree of concordance between the two registers has been proven for malignant diseases, with evidence of completeness of registration approaching 95% [Citation8].
The aim of this study is, by access to both registers and a meticulous review of individual electronic patient records, to present a true population-based study of incidence, stage distributions, Lauren distributions, resection rates and long-term survival rates for patients with gastric adenocarcinoma in Central Norway during the period 2001–2011.
Material and methods
Study population
Central Norway comprises the three counties of Møre og Romsdal, Sør-Trøndelag, and Nord-Trøndelag. The population increased from 635 936 in 2001 to 680 110 in 2011, representing 14.1% and 13.8%, respectively, of the total Norwegian population. Care of gastric cancer in the early part of the study period was divided between eight hospitals in Central Norway, but as of 2004 has been centralized to St. Olavs Hospital, the university hospital of the region.
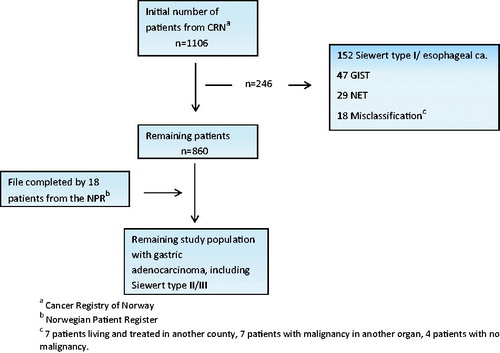
An initial search in the CRN database was conducted for the period 2001–2011 using ICD-10 codes C16.1 to C16.9 and C15.5/C15.9. The list of patients accrued was completed through a similar search in the NPR database. Through access to individual electronic patient records, patients confirmed to have a diagnosis of adenocarcinoma were retained, whereas patients with gastric tumors of other origin, or misclassified entries in the CRN or NPR records, were excluded from further analysis. For tumors at the gastric cardia, only patients harboring types II or III cancers according to the classification by Siewert [Citation9] were retained (). The type I cancers for the last decades have been staged and treated as distal esophageal cancers, and were not part of the objective of the present study.
Figure 1. Flowchart identifying the final population-based study cohort of gastric adenocarcinoma in Central Norway 2001–2011.
A dedicated pathologist reviewed the microscopic specimens to secure a uniform histological classification according to Lauren [Citation10] and T-staging according to the seventh edition of the UICC classification for gastric cancer [Citation11]. The resection status of R0, R1 or R2 was used as defined by the UICC [Citation11].
Since March 2007, medically fit patients below 75 years of age with resectable gastric cancer have been offered a MAGIC style regimen of perioperative chemotherapy [Citation12]. Ninety-five patients included in the present study received this treatment. Response rates and oncological outcomes were reported in a recent paper [Citation13]. In the present paper, the disease stage for those who proceeded to surgery (n = 84) is reported as ypTN. For patients not operated on (n = 11) due to progressive disease or deteriorating medical conditions following chemotherapy, the disease stage is reported as concluded from initial computed tomography (CT) scans.
To enable comparison of the outcomes across different populations or across different time periods, age-specific and world age-standardized incidence rates are provided [Citation14].
To reveal any time trends, the study period was divided into two halves, from 2001 to 2006 and 2007 to 2011, respectively. The censoring date was 31 December 2015, allowing for a minimum follow-up of four years.
Statistics
Continuous data are presented as median (range) and compared by the Mann-Whitney U-test. Categorical variables were analyzed using the χ2-test. Long-term overall survival was calculated from the time of diagnosis and estimated by the Kaplan-Meier (K-M) method, and compared using the log-rank test. Furthermore, a conditional survival was estimated by excluding patients that died within three months of diagnosis, irrespective of cause. Net survival was estimated as relative survival, applying the method described by Pohar-Perme [Citation15], using population lifetables obtained from Statistics Norway, stratified on sex, age and calendar year. Effect measures are presented with 95% confidence intervals (CI). All tests were two-sided with the level of significance set to 0.05. Analyses were done using SPSS® version 23 (IBM, Armonk) and Stata-14 (StataCorp 2015).
Results
Patient population
A total of 1106 patients were identified through the initial comprehensive search of the CRN. Of these, 246 did not meet the inclusion criteria, and were excluded from further analysis. The list of the 860 remaining patients was supplemented by 18 patients identified through the NPR search for the years 2008 to 2011, providing 878 patients accumulated over 11 years to constitute the study population, 488 in the early time period and 390 in the late one (). This implies an average annual crude incidence of 15.6/105 for males and 9.1/105 for females, and world age-standardized incidence of 8.0/105 and 3.6/105, respectively. Only one patient was lost to follow-up.
The diagnosis of gastric adenocarcinoma was histologically verified for 846 patients (96.4%). The remaining 32 patients (3.6%) had their diagnosis established merely through findings at gastroscopy and/or at CT. Among these, 18 were found to have organ metastases (M1) and nine to be in an M0 situation, while five had no attempts at staging. The study population comprised 555 males (63.2%) and 323 females (36.8%). The median age was 76.2 years (27.1–99.4), and was significantly lower for males (74.5 years) compared to females (78.2 years) (p < .001). The median age for males was 75.8 years in the early time period compared to 71.9 during the later one (p = .058). For females, no such trend was seen, with median age 78.0 years and 78.4 years, respectively.
The crude male to female ratio was 1.72. For the ages between 50 and 70 years the ratio exceeded 2.0, while remaining closer to unity for both the younger and older patients. For the 166 patients above 85 years the ratio was even inversed with 46% males and 54% females, attributed to more females at risk. This pattern was modified for the incidence rates, providing a ratio of approximately 2.30 irrespective of age, except for the category below 50 years of age ().
Table 1. Population-based demographics, tumor location, stage distribution, treatment, and long-term survival of gastric adenocarcinoma in Central Norway 2001–2011 stratified by age, n = 878.
Lauren classification
The 846 patients with histologic proven gastric adenocarcinoma had a Lauren distribution of 434 patients (51.3%) with intestinal type, 267 (31.6%) with diffuse type, and 106 (12.5%) with a mixed type (). Thirty-nine patients (4.6%) had an unspecified adenocarcinoma according to this scheme. The proportion of diffuse type was significantly higher among females (41%) than males (25%) (p < .001), for age below 60 years (55%) compared to age above 60 years (29%) (p < .001) and for non-cardia cancers (36%) compared to cardia cancers (18%) (p < .001). For the Lauren intestinal type, the proportion of tumors located at the gastric cardia was strongly dependent upon age. It decreased steadily from 72% among the few patients with the intestinal type and age below 60 years, to 37% among the patients aged 60–80 years, and down at 20% among the large group of patients aged 80 years or more. That is, elderly patients had their intestinal type of cancer predominantly distally located. The Lauren mixed type accounted for 13.1% of the 807 patients with a specified Lauren classification, and 12.1% of all patients included in this study. The proportion was rather constant at around 11–14% irrespective of age category or sex, although with a slightly higher proportion among males ().
Table 2. Population-based demographics, tumor location, stage distribution, treatment, and long-term survival of gastric adenocarcinoma in Central Norway 2001–2011 stratified by Lauren classification, n = 807.
Tumor location
Patients with tumors at the gastric cardia were younger than the median age of the study cohort (). They harbored a strong male dominance with a distinct preponderance for type II cancers, whereas females were relatively more often diagnosed with the type III subtype (data not shown). Males had 33.5% of their cancers located at the gastric cardia, with a significant increase from 29.7% in the early study period to 39.1% in the later (p = .005). Females had 11.8% of their cancers at the gastric cardia, with no difference across time periods.
Table 3. Population-based demographics, Lauren category, stage distribution, treatment, and long-term survival of gastric adenocarcinoma in Central Norway 2001–2011 stratified by tumor location, n = 878Table Footnotea.
Females had a substantial proportion of around 16% of their cancers diffusely located, largely because of a high frequency of the Lauren diffuse type. On an absolute scale, the number of diffusely located cancers equaled that of the males ().
Gastric remnant cancers still represented a significant proportion. They accounted for 7.9% of all cases (69 patients), 10% among the male cases compared to 5% among the females (p = .006).
Treatment
Overall, 341 patients (38.8%) had an R0-R1 resection and 54 patients (6.2%) an R2 resection, whereas 483 patients (55%) had no resection done (). The resections included 28 proximal resections, 154 total gastrectomies, 209 subtotal/distal resections, and four wedge resections. Age-stratified resection rates and outcomes are reported in .
No difference was found in the proportion of R0-R1 resections across the two time periods, 39.1% compared to 38.5%. Females had a non-significant trend towards a lower R0-R1 resection rate (35.0%) compared to males (41.1%) (p = .074). This may be due to a higher proportion of diffusely located cancers among females, and a higher proportion of elderly and frail patients among the females.
The treatment provided for the 483 patients with no resection was a gastro-jejunostomy in 31 (3.5%), explorative laparotomy in 42 (4.8%) and primary stent in 47 (5.4%), whereas 363 (41.3%) had no surgical measure. The group of patients with no resection had a median age of 79.2 years. It included 325 patients (67.3%) staged to M + disease with a median age of 75.3 years, and 158 patients (32.7%) staged to M0 (105 patients) or Mx (53 patients) with a median age of 84.6 years.
Overall resection rates were not correlated to the Lauren categories. This belied the fact that patients with the Lauren diffuse type more often presented with metastatic disease, 131 patients (49.1%) compared to 162 patients (37.3%) for the intestinal type (p = .011). However, the intestinal type was more frequent among the elderly not subjected to surgery due to advanced comorbidity. Hence, both Lauren main categories had a substantial proportion of patients that did not receive resection, albeit for quite different explanatory reasons.
Stage distribution
In 752 (85.6%) patients a conclusive staging could be performed either by examination of the resected specimen (n = 383), by CT scan and findings at non-resection laparotomy (n = 64) or at CT scan alone (n = 305). In the latter group, 268 patients were found to be in an M + situation ().
Table 4. Population-based stage distribution for gastric adenocarcinoma in Central Norway 2001–2011 (TNM 7th edition), with corresponding median and long-term overall survival rates, n = 878.
Among the remaining 126 (14.4%) patients with disease stage X, 73 were judged through initial CT staging (n = 52) and/or findings at laparotomy (n = 21) to be in an M0 situation. Fifty-three patients remained in an Mx situation as no staging was ever attempted.
The 341 patients with an R0-R1 situation had a median of 10 lymph nodes (range 0–91) harvested; their distribution by node category was 168 N0 (49.3%), 57 N1 (16.7%), 51 N2 (15.0%), 55 N3 (16.1%) and 10 Nx.
Early gastric cancer (EGC) is defined as a T1 cancer [Citation16], although carcinoma in situ (Tis) are often included in the reported numbers [Citation17]. A very small percentage of advanced cancers subjected to chemotherapy were downstaged to ypT0/T1. Although reported with their ypTN stage in , they are not counted as EGC in the following discussion. The number of true EGCs was hence found to be 73 (8.3%), counting nine Tis and 64 T1 cancers, 28 mucosal (one N1), 34 submucosal (one N1, two N2) and two of unspecified depth of growth. Their median age was 76.6 years (43–90), with 73% of the cases detected in males and 27% in females, corresponding to a sex ratio of 2.7, which is well above the corresponding ratio for the total study population. Among males EGC constituted 9.5% of all cases and among females 6.2%, with the proportions highly dependent on age at diagnosis (), Lauren classification () and tumor location (). The Lauren distribution among the 72 EGC patients with a defined category was 70.8% intestinal, 19.4% diffuse and 9.7% mixed type.
Of 341 patients with an R0-R1 resection, 69 (20.2%) had EGC. The five-year overall survival for the 28 patients with growth limited to the mucosa (T1a) was 82.1% (95% CI 68.0–96.2) and for the 34 patients with submucosal invasion (T1b) 73.5% (95% CI 58.6–88.4) (log-rank p = 0.039).
Long-term survival rates
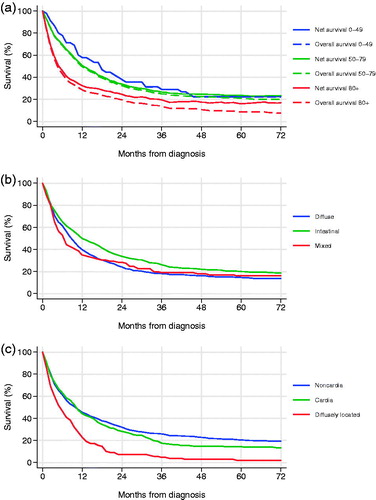
The median survival of all included patients was 9.0 months (95% CI 7.8–10.2), five-year overall survival 16.3% (95% CI 13.8–18.8) and five-year net survival 20.6% (95% CI 17.4–24.1). Five-year overall survival stratified by age (), Lauren type (), tumor location (), and by stage () are presented. Overall and net survival K-M curves stratified by age are depicted in . Conditional survival was calculated by excluding patients that died within three months of diagnosis irrespective of cause. The 656 patients remaining (75% of the original study cohort) had a median survival of 15.6 months (95% CI 13.5–17.7) and a five-year overall survival of 21.9% (95% CI 18.8–25.0).
Figure 2. Population-based survival of gastric adenocarcinoma in Central Norway 2001–2011 (a) overall and net survival by age category, (b) overall survival by Lauren type, (c) overall survival by tumor location.
Median survival for R0-R1 resected patients was 37.6 months (95% CI 29.6–45.6), with a five-year overall survival of 40.9% (95% CI 35.6–46.2). Median survival for patients who received an R2 resection was 8.0 months (95% CI 6.2–9.8), and for those not subjected to resection 3.6 months (95% CI 3.2–4.1). Among patients who did not receive any resection, nine survived beyond three years, whereas none of the patients with R2 surgery did so. The groups, however, are not similar. The first group may include patients with a low tumor burden, yet not subjected to surgery for medical reasons.
Survival for patients with the Lauren intestinal type was superior to that of the diffuse type (log-rank p = .033), although compared to the mixed type the difference was not statistically significant (log-rank p = .087) (). Limiting the survival analysis to the 336 patients with a defined Lauren status and an R0-R1 resection, there was no longer any difference between the intestinal and the diffuse type (log-rank p = .129).
There was no survival difference between the cancers at the gastric cardia compared to the non-cardia cancers (log-rank p = 0.171). However, both tumor locations had superior survivals (log-rank p < .001) compared to the diffusely located cancers that exhibited a high proportion of patients diagnosed with metastatic disease and a subsequent R0-R1 resection rate of only 14% ().
Considering all included patients, no trend for survival differences across the early (2001–2006) and the late (2007–2011) time periods were found, even when constraining analysis to the patients that had an R0-R1 resection, (log-rank p = 0.091). Among patients that received R0-R1 surgery and that later experienced relapse, 26% were treated with palliative chemotherapy, with no trend across the study period. For patients subjected to R2 surgery or less, 29% received palliative chemotherapy as part of primary treatment, with no trend across the study period.
Discussion
A distinct feature of this study compared to other population-based series is that each patient was individually accessed through the electronic patient records, and that the study was conducted within a strictly defined geographical area with public censuses available, allowing for incidence rates to be computed. Only one patient was lost to follow-up, a testimony to a limited migration among the elderly in Norway and to a transparent community, features perhaps only conceivable in a Scandinavian population.
The most important finding of this study was a very low five-year overall survival rate of 16% and an R0-R1 resection rate of 39%. This is substantially inferior to what is typically reported from hospital materials, and to a large extent consequent upon a high proportion of 44% of the patients diagnosed with stage IV disease, and 38% of the patients aged 80 years or more. Six per cent of the patients were too frail to be considered for adequate staging and around a quarter of the patients in an M0 situation were unable to receive surgery due to a combination of advanced disease, high age and comorbidity. A low proportion of only 8% had EGC. These findings are to some extent familiar from hospital based materials [Citation18,Citation19], but the severity is more clearly disclosed through population-based studies like the present one.
The age-stratified analysis reveals a very different situation for the two-thirds of the patients below 80 years. Although a high proportion of 46.7% with stage IV disease is still encountered, virtually none are left without staging and merely 12% of the M0 patients did not receive resections. This translates into an R0-R1 resection rate of 47.1%, which is substantially higher than the 25.2% observed for patients above 80 years, and is followed by a five-year overall survival of 44.0% for this subset of patients, compared to 31.3% for R0-R1 resected patients above 80 years of age (). The findings are mirrored in a conditional five-year overall survival of 21.9% for those surviving the first three months, and a long-term net survival distinctly above the overall survival for patients above 80 years ().
A superior survival rate was documented for the Lauren intestinal type compared to the diffuse type, although not significant when limited to the patients with R0-R1 resections. By such a restriction, a large group of Lauren diffuse type tumors with metastasis was eliminated along with a high proportion of old patients with the intestinal type of cancer, not operated for medical reasons. These findings may explain the relative paucity of documentation of superior survival for the intestinal subtype, as hospital-based series might not be able to catch the full range of patients.
For males, a significant increase was documented in the proportion of cancers at the gastric cardia, from 29.7% in the first time period to 39.1% in the later. This was largely accounted for by somewhat younger male patients although in their middle age, and explains the substantial decrease in the median age for males seen across the study period. This is of particular note, as type I cardia cancer was not included in this study. This implies that the type II cancer, which in this study by far was the most prevalent type among males, even within this short time span is embraced by the sharp rise of proximal gastric cancers encountered worldwide. This rise is considered to be caused by epidemic overweight and years of chronic gastro-esophageal reflux [Citation20,Citation21].
For some variables, such as the crude sex ratio, the proportion of cardiac cancers and the five-year survival rates, results seem to peak at the age groups of 50–70 years (). Patients below the age of 50 in some respects proved more similar to the patients above 80 [Citation6,Citation22]. A crude male to female ratio of 1.3 among older patients, as compared to 2.4–2.3 for the age categories of 50–70 years, is readily explained by the fact that females were more prevalent among the octogenarians. For patients below 50 years of age, the Lauren diffuse type was dominant, explaining a crude ratio of 1.6. Concerning incidence rates, however, a quite stable ratio around 2.3 for the age categories above 50 years are found, suggesting that with increasing age, females maintain a stable relative risk compared to males. For patients below 50 years of age a ratio of 1.5 is found even for the incidence rates ().
The tendency for the proportion of type II and III cardia cancers and five-year survival rates to peak around the middle age categories can be understood by looking at the different Lauren types separately (). The Lauren diffuse type was dominant among the patients below 60 years of age, leaving less than a third to be accounted for by the intestinal type. It had a low proportion of cardia cancers, was more often diffusely located and with a very high rate of metastatic disease. The Lauren intestinal type, however, only started to rise after the age of 60 and accounted for more than two-thirds of the patients above 80 years. However, the proportion of cardia cancers among the Lauren intestinal type monotonously decreased with age. For the patients above 80 years it merely constituted 20%. These two patterns of Lauren distribution combined to give a relative infrequency of cardia cancers among the youngest and the oldest patients. The cardia cancers peaked around the middle age groups where the intestinal type was at the rise, whereas the tendency for a distal tumor location following older age had yet to make its impact. Likewise, these data combined to explain the inferior long-term survival among the younger patients having predominantly advanced Lauren diffuse disease, and among the older patients having predominantly intestinal disease, yet often not operated on for medical reasons.
At the outset, Lauren attempted a classification with only two distinct groups, leaving the remainder to an indeterminate group. Over the years this group has been substantiated as a distinct histological entity, termed the mixed type. The findings of the present study are in line with this development. The mixed type accounted for a stable proportion of around 11–14%, irrespective of age and sex category, although for several other variables such as median age, frequency of EGC, tumor location, proportion of M + and R0-R1 resection rates, it took on values clearly intermediate to that of the intestinal and diffuse types. Median survival seems inferior, with the K-M curve close to that of the Lauren diffuse one (). This observation has been reported by others, and is attributed to a tendency for the mixed type to show deep wall penetration with a high proportion of N + tumors [Citation23,Citation24]. Future improvements in molecular and genetic profiling of tumors might make it possible to retain merely two categories, but at present the conception of the mixed type as a distinct entity can be well argued, both from an epidemiological and a clinical point of view.
A limitation of the present study is that it constitutes only 14% of the total Norwegian population. However, there are reasons to believe that the findings are representative of that of the national population during the relevant time period. This contention is based on an age distribution in Central Norway that is similar to the national one, and a percentage of females versus males that for each age stratum equals that of the national population. Furthermore, the number of cases in Central Norway reported in the present study is proportionate to the population basis, accounting for 14.9% of the total national caseload. For the same time period the population of Central Norway on an average constituted 14.0% of the national population. And finally, on a national scale the proportion of patients reported by the CRN to be diagnosed in a localized stage (N0, M0) was 20.7%, in a regional stage (N+, M0) 27.9% and in an M+/unknown situation 51.5%. Corresponding numbers for Central Norway in the present study are 19.1% in a localized stage, 31.2% in a regional stage and 49.7% in an M+/unknown situation.
Disclaimer
“The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred.”
“Data from the Norwegian Patient Register has been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian Patient Register is intended nor should be inferred.”
Acknowledgments
We would like to acknowledge the skillful help provided by the pathologists Dr. L. Matuszkiewicz (Molde hospital) and Dr. B. Westre (Ålesund Central Hospital) who reviewed some of the microscopic specimens for a definite Lauren classification.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86.
- Cancer Registry of Norway 2016. www.kreftregisteret.no/no/Registrene/kreftstatistikk/
- Kunz PL, Gubens M, Fisher GA, et al. Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol 2012;30:3507–15.
- Schlesinger-Raab A, Mihaljevic AL, Egert S, et al. Outcome of gastric cancer in the elderly: a population-based evaluation of the Munich Cancer Registry. Gastric Cancer 2016;19:713–22.
- Chapelle N, Manfredi S, Lepage C, et al. Trends in gastric cancer incidence: a period and birth cohort analysis in a well-defined French population. Gastric Cancer 2016;19:508–14.
- Caruso R, Irato E, Branca G, et al. Gastric adenocarcinoma incidence in the province of Messina (Insular Italy): a cancer registry study. Oncol Lett 2014;7:861–5.
- Dassen AE, Lemmens VE, van de Poll-Franse LV, et al. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: a population-based study in the Netherlands. Eur J Cancer 2010;46:1101–10.
- Bakken IJ, Gystad SO, Christensen ØO, et al. Comparison of data between the Norwegian Patient Register and the Cancer Registry of Norway. Tidsskrift Nor Laegeforen 2012;132:1336–40.
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457–9.
- Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal type carcinoma. An attempt at a histological classification. Acta Pathol Microbiol Scand 1965;64:31–43.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th ed. Chichester (UK): Wiley-Blackwell; 2009.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20.
- Bringeland EA, Wasmuth HH, Fougner R, et al. Impact of perioperative chemotherapy on oncological outcomes after gastric cancer surgery. Br J Surg 2014;101:1712–20.
- Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical report. Berlin: Springer-Verlag; 1966.
- Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics 2012;68:113–20.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12.
- Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol 2001;36:445–56.
- Fuentes E, Ahmad R, Hong TS, et al. The impact of neoadjuvant therapy for gastroesophageal adenocarcinoma on postoperative morbidity and mortality. J Surg Oncol 2016;113:560–4.
- Wang W, Zheng C, Fang C, et al. Time trends of clinicopathologic features and surgical treatment for gastric cancer: results from 2 high-volume institutions in southern China. Surgery 2015;158:1590–7.
- Lagergren J, Bergstrøm R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999;130:883–90.
- Lagergren J, Bergstrøm R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
- Rutegård M, Shore R, Lu Y, et al. Sex differences in the incidence of gastrointestinal adenocarcinoma in Sweden 1970-2006. Eur J Cancer 2010;46:1093–100.
- Stelzner S, Emmrich P. The mixed type in Laurén's classification of gastric carcinoma. Histologic description and biologic behavior. Gen Diagn Pathol 1997;143:39–48.
- Zheng H-C, Li X-H, Hara T, et al. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch 2008;452:525–34.
