Abstract
Background: Pazopanib is a multitargeted tyrosine kinase inhibitor approved for the treatment of patients with selective subtypes of advanced soft tissue sarcoma (STS) who have previously received standard chemotherapy including anthracyclines. Data on the efficacy in vascular sarcomas are limited. The main objective of this study was to investigate the activity of pazopanib in vascular sarcomas.
Patients and methods: A retrospective study of patients with advanced vascular sarcomas, including angiosarcoma (AS), epithelioid hemangioendothelioma (HE) and intimal sarcoma (IS) treated with pazopanib in real life practice at EORTC centers as well as patients treated within the EORTC phase II and III clinical trials (62043/62072) was performed. Patient and tumor characteristics were collected. Response was assessed according to RECIST 1.1. and survival analysis was performed.
Results: Fifty-two patients were identified, 40 (76.9%), 10 (19.2%) and two (3.8%) with AS, HE and IS, respectively. The response rate was eight (20%), two (20%) and two (100%) in the AS, HE and IS subtypes, respectively. There was no significant difference in response rate between cutaneous and non-cutaneous AS and similarly between radiation-associated and non-radiation-associated AS. Median progression-free survival (PFS) and median overall survival (OS; from commencing pazopanib) were three months (95% CI 2.1–4.4) and 9.9 months (95% CI 6.5–11.3) in AS, respectively.
Conclusion: The activity of pazopanib in AS is comparable to its reported activity in other STS subtypes. In this study, the activity of pazopanib was similar in cutaneous/non-cutaneous and in radiation/non-radiation-associated AS. In addition, pazopanib showed promising activity in HE and IS, worthy of further evaluation.
Soft tissue sarcomas (STS) represent a highly heterogeneous group of tumors with differing underlying biology, clinical behavior and response to systemic therapy [Citation1]. Within this family of tumors, vascular sarcomas such as angiosarcoma (AS), epithelioid hemangioendothelioma (HE) and intimal sarcoma (IS), account for about 2–3% of adult STS [Citation2]. The worldwide age-standardized incidence rate of AS is approximately 0.1/100000/year [Citation3].
There are few prospective data on the utility of systemic therapy in vascular sarcomas. The majority of prospective trials evaluating systemic therapies in sarcomas have generally included all or many STS subtypes in a one size fits all approach. Consequently, the number of patients with vascular sarcomas in such trials is low. Based on retrospective data, standard first- and second-line therapy for AS include doxorubicin- and taxane-based regimens. Furthermore, there have been a number of phase II trials in AS. The phase II AngioTax trial reported a response rate to weekly paclitaxel of 20% with a disease stabilization rate of 75% after three months and 24% after six months [Citation4]. First-line single-agent doxorubicin and weekly paclitaxel seem to have similar efficacy in metastatic AS [Citation5,Citation6]. First-line anthracycline-based chemotherapy was associated with a partial response rate of 25% and a median progression-free survival (PFS) of 4.9 months [Citation7].
Vascular sarcomas are known to express pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) [Citation8]. Therefore, the evaluation of vascular-targeted agents in vascular tumors such as AS, HE and IS is of particular interest. Accordingly, bevacizumab was investigated for the treatment of AS and HE confirming promising efficacy results represented by a median PFS of 12 and 52.7 weeks, respectively [Citation9]. In two phase II trials by Maki and Ray-Coquard and colleagues, a median PFS in the range of 1.8–3.8 months, respectively, was reported for sorafenib in AS [Citation10,Citation11].
Pazopanib is a tyrosine kinase inhibitor targeting several factors including VEGFR1-3 and PDGFR and has been approved by the European Medicines Agency (EMA) for the treatment of patients with advanced non-adipocytic STS who have previously been treated with anthracycline- or ifosfamide-based chemotherapy [Citation12]. There are few published data on the efficacy of pazopanib in vascular sarcomas [Citation13]. Due to the small number of patients with vascular sarcomas included in the EORTC STS phase II and III trials, a meaningful evaluation of its activity in these subtypes is not possible [Citation14,Citation15]. There have been case reports and small case series documenting the potential efficacy of pazopanib in vascular sarcomas [Citation16–19]. In addition, pazopanib is the only tyrosine kinase inhibitor approved for non-adipocytic STS together with imatinib in DFSP, and therefore further evaluation of this agent in vascular sarcomas has an added value. Consequently, the aim of this study was to document the efficacy of pazopanib in a cohort of patients with advanced vascular sarcomas treated at a number of EORTC centers and within the two EORTC trials, in order to provide a benchmark for further studies and clinical practice.
Material and methods
Patient population and data collection
Ethical approval was obtained according to local and national regulations. A retrospective search of patients with advanced vascular sarcomas treated with pazopanib at EORTC-STBSG centers was performed. In addition, patients with vascular sarcomas treated within the phase II and III EORTC pazopanib trials (62043/62072) were identified. Patients were included in the analysis regardless of treatment line. In some patients pazopanib was given in the first-line setting as pazopanib is reimbursed in several countries as first-line therapy if patients are not suitable for treatment with anthracyclines. Pazopanib was administered according to standard ESMO Guidelines and local institutional policy. Clinical, pathological and outcome data were obtained from the individual patient records and from the EORTC database for the patients treated within trials. The diagnosis was confirmed in all cases by an experienced STS pathologist. All patients (but one) were confirmed to have progressive disease before treatment start.
Endpoints
The study endpoints were response rate to pazopanib, PFS and overall survival (OS). The objective response rate was assessed per RECIST 1.1. The disease control rate was defined as the sum of complete response, partial response and stable disease. PFS was defined from the date of commencing pazopanib to the first documentation of progression or death. For data obtained from the EORTC trials, the definition of progression was according to the radiological assessment of the principal investigator; in the absence of radiologically documented progression, clinical progression was also taken into account.
OS was calculated from the starting date of pazopanib administration to the date of death. Patients alive at the time of analysis were censored at the date of last follow-up. For patients with AS treated with pazopanib, the outcome of those with cutaneous and non-cutaneous types was compared, and likewise for radiation-associated and non-radiation-associated.
Statistical analysis
Descriptive summary statistics of patients, tumor and treatment characteristics, and response to treatment are provided overall as well as stratified by the histologic subtypes of vascular sarcoma (AS, HE and IS). Furthermore, the response rate of AS was determined by origin of the primary tumor (cutaneous/non-cutaneous) and by association with radiation therapy (Yes, No), and tested for association with these covariates using a χ2-test.
Survival curves for PFS and OS were generated using the Kaplan–Meier method for the different histologic subtypes. Estimates of medians and corresponding 95% confidence intervals (CI) are reported.
Results
Patient and tumor characteristics
A total of 52 patients were identified, including nine patients from the EORTC phase II and III trials (62043 and 62072). Sixteen institutions in eight countries contributed to this analysis. Baseline patient and tumor characteristics are summarized in . Among the vascular sarcoma patients included, 40 (76.9%) had AS, 10 (19.2%) had HE and two (3.8%) had IS. Focusing on the AS cohort the overall median age at diagnosis was 62.4 years (range 30–83.4 years). A total of 62.5% of patients were male. The most common primary tumor site was breast (n = 15) and scalp (n = 6). Additionally, AS was diagnosed in the abdomen (adrenal gland, liver, genitourinary) (n = 8), chest (including pulmonary artery) (n = 7) and extremities (n = 4). Twenty-four patients (60%) had non-cutaneous AS and 14 patients (35%) had radiation-associated AS.
Table 1. Patient and tumor characteristics of vascular sarcomas.
Treatment details before and after pazopanib
In five patients (9.6%) pazopanib was given in the first-line setting (). The most commonly administered previous treatments were paclitaxel (54.5%, n = 25) and doxorubicin (43.5%, n = 20). The details of other treatments administered are displayed in Supplementary Table 1 (available online at http://www.informahealthcare.com).
Of the patients on follow-up at the time of analysis, 21 (48.8%) received no additional therapy, 18 (41.9%) received one further line of therapy and four patients (9.3%) received two further lines of therapy (). The most commonly administered drugs following progressive disease on pazopanib were gemcitabine (21.1%, n = 8) and paclitaxel (18.4%, n = 7). Details for the other therapies administered are provided in Supplementary Table 1.
Clinical outcome and prognostic factors
All patients (but one) were confirmed to have progressive disease before treatment start. The median follow-up was 15.9 months (95% CI 12.8–38.2), and it was not possible to obtain response data for three patients (5.8%). Overall, 26 patients (50%) experienced progressive disease, 11 patients (21.2%) had stable disease, 11 patients (21.2%) a partial response and one patient (1.9%) had a complete response (), leading to a disease control rate for the total group of 44.3%.
Table 2. Treatment details before and after pazopanib.
In the AS cohort, the rate of partial response and stable disease was 20% and 17.5%, respectively, representing a clinical benefit rate of 37.5% (). The response rate was similar in cutaneous and non-cutaneous AS, 26.7% and 16.7% (p = 0.54), respectively. Furthermore, the response rate was 28.6% in the radiation-associated cohort and 15.4% in the non-radiation-associated AS cohort (p = 0.39), respectively ().
Table 3. Best response to pazopanib.
Table 4. Response rate (CR and PR) according to RECIST 1.1. For angiosarcoma patients.
In the cohort of patients with HE, the clinical benefit rate was 60% of which 67% (i.e. 4 of 6) experienced disease stabilization as the best response. Of note, both patients with IS had a partial response ().
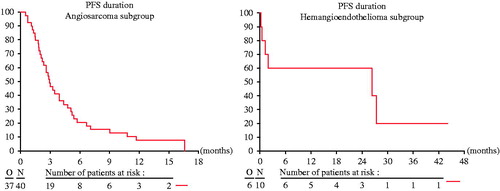
The median PFS was three months (95% CI 2.1–4.4) in the AS cohort and 26.3 months (0.2–N) in the HE cohort, respectively (). The median OS from date of treatment start was 9.9 months (95% CI 6.5–11.3) and 26.3 months (95% CI 0.5–N) for AS and HE, respectively.
Discussion
STS represent a group of rare and heterogeneous tumors of mesenchymal origin, with each subtype having differing underlying biology, clinical behavior and response to systemic therapy. In view of the rarity of these tumors they have often been grouped together for the purpose of clinical trials, and consequently the results of such trials have often been difficult to interpret with regard to the benefit of an individual drug or schedule in a specific subtype. We aimed to get more information on a couple of vascular sarcomas in patients treated with pazopanib within the first clinical trials and in daily clinical practice after registration.
Our study documented a disease control rate of 40% and a median PFS of three months for AS patients treated with pazopanib. These results are consistent with the phase II data of sorafenib in AS [Citation10]. In the PALETTE trial, the median PFS of 4.6 months was slightly higher in the STS group overall [Citation15]. Up-regulation of angiogenic growth factors is thought to play a major role in the pathomechanism of several tumors, including ASs. In the latter Itakura et al. report on an immunohistochemical expression of certain VEGFs and their receptors in over 90% of patients [Citation20]. Therefore, one would expect a higher response rate to angiogenic drugs in AS patients when compared to other STS. However, our results do not support this assumption. It should be noted that the patients in our cohort were heavily pretreated, with 25% having received more than two lines of systemic therapy prior to commencing pazopanib. The most commonly employed chemotherapy drugs were paclitaxel, gemcitabine and liposomal doxorubicin.
Several retrospective studies suggest that cutaneous AS is a relatively chemosensitive subtype, particularly to paclitaxel, but data on targeted agents are lacking [Citation21]. However, in our cohort no significant difference in the efficacy of pazopanib was observed between patients with primary cutaneous and non-cutaneous primary tumor sites. Radiation-associated AS is known to express several angiogenic biomarkers (i.e. VEGF) supporting the rationale for angiogenesis inhibitors in patients with these tumors [Citation8]. However, no significant difference in response rate was observed between radiation-associated and non-radiation-associated AS, but the number of patients is in fact too small to draw firm conclusions.
HE has a spectrum of behavior from indolent to very aggressive. Overexpression of vascular growth factor receptors (VEGF, VEGFR2, and VEGFR3) has been observed in pulmonary HE samples [Citation22]. Given its vascular origin, angiogenesis inhibition may be a reasonable therapeutic approach for the management of this subtype. Interferon-2, which has some anti-angiogenic activity, has been reported to result in responses in some case reports [Citation23]. In the trial by Agulnik et al. the efficacy of bevacizumab was assessed in HE and AS. Seven patients with HE were included; two had a partial response and four had stable disease [Citation9]. A phase II trial of sorafenib by the French Sarcoma Group included 15 patients with HE; two patients had a partial response and five SD [Citation24]. Our results are consistent with the French Sarcoma Group trial, and demonstrate that pazopanib can result in RECIST responses in some patients with HE. Additionally, our survival data are consistent with the reported mean survival of approximately 4.6 years including a broad range from six months to 24 years [Citation25]. However, all of these observations are limited by the small patient numbers due to the rarity of this disease.
There are few published data regarding systemic therapy in IS, although patients are often treated with anthracyclines and ifosfamide [Citation26]. To our knowledge no data of antiangiogenic therapy is available in this subtype. In view of both patients achieving a partial response to pazopanib further evaluation is warranted in this subtype.
Our study is limited by its retrospective nature and small sample size. However, to our knowledge, this report represents the largest published series of patients with advanced vascular sarcomas treated with pazopanib. Our study suggests that pazopanib has activity in AS, but the responses observed in patients with HE and IS warrant further investigation. Investigating potential predictive markers on a molecular level for suggested differences in treatment sensitivity between AS, HE or IS, respectively, or between pazopanib responders and non-responders in the AS cohort would be of major interest.
IONC_1234608_supplementary.docx
Download MS Word (17.4 KB)Acknowledgments
We would like to thank the patients who participated in the EORTC-STBSG clinical trials. We are also grateful to Saskia Litiere and Nathan Touati for their contributions to the statistical analysis.
Disclosure statement
Attila Kollár had a consultant/ advisory role with Novartis. Silvia Stacchiotti received research funding from Novartis. Giovanni Grignani received research funding from GSK and Novartis. Winette van der Graaf received research funding from GSK and Novartis. Bernd Kasper received honoraria from Novartis.
References
- Fletcher CDM, Hogendoorn P, Mertens F. eds. WHO classification of tumors of soft tissue and bone (IARC WHO Classification of Tumors). 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2013.
- Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
- Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011;6:e20294.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol 2008;26:5269–74.
- Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer 2005;104:361–6.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
- Young RJ, Natukunda A, Litiere S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer 2014;50:3178–86.
- Azzariti A, Porcelli L, Mangia A, et al. Irradiation-induced angiosarcoma and anti-angiogenic therapy: a therapeutic hope?. Exp Cell Res 2014;321:240–7.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 2013;24:257–63.
- Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO). Oncologist 2012;17:260–6.
- Maki RG, D'adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009;27:3133–40.
- Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol 2011;77:163–71.
- Kasper B, Hohenberger P. Pazopanib: a promising new agent in the treatment of soft tissue sarcomas. Future Oncol 2011;7:1373–83.
- Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organization for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–32.
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
- Yoo KH, Kim HS, Lee SJ, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer 2015;15:154.
- Fujita M, Endo Y, Fujisawa A, et al. Pazopanib: an alternative in taxane-resistant cutaneous angiosarcoma. Eur J Dermatol 2014;24:267–8.
- Tomita H, Koike Y, Asai M, et al. Angiosarcoma of the scalp successfully treated with pazopanib. J Am Acad Dermatol 2014;70:e19–21.
- Kasper B, Sleijfer S, Litiere S, et al. Long-term responders and survivors on pazopanib for advanced soft tissue sarcomas: subanalysis of two European Organization for Research and Treatment of Cancer (EORTC) clinical trials 62043 and 62072. Ann Oncol 2014;25:719–24.
- Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. Journal of Surgical Oncology 2008;97:74–81.
- Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer 2008;44:2433–6.
- Stacher E, Gruber-Mosenbacher U, Halbwedl I, et al. The VEGF-system in primary pulmonary angiosarcomas and haemangioendotheliomas: new potential therapeutic targets?. Lung Cancer 2009;65:49–55.
- Radzikowska E, Szczepulska-Wojcik E, Chabowski M, et al. Pulmonary epithelioid haemangioendothelioma-interferon 2-alpha treatment-case report. Pneumonol Alergol Pol 2008;76:281–5.
- Chevreau C, Le Cesne A, Ray-Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO). Cancer 2013;119:2639–44.
- Sardaro A, Bardoscia L, Petruzzelli MF, et al. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014;8:259.
- Uchida A, Tabata M, Kiura K, et al. Successful treatment of pulmonary artery sarcoma by a two-drug combination chemotherapy consisting of ifosfamide and epirubicin. Jpn J Clin Oncol 2005;35:417–19.