Abstract
Background: Neoadjuvant chemoradiation therapy (CRT) increases pathological complete response (pCR) rates compared to radiotherapy alone in patients with stage II-III rectal cancer. Limited evidence addresses whether radiotherapy dose escalation further improves pCR rates. Our purpose is to measure the effects of radiotherapy dose and other factors on post-therapy pathologic tumor (ypT) and nodal stage in rectal cancer patients treated with neoadjuvant CRT followed by mesorectal excision.
Material and methods: A non-randomized comparative effectiveness analysis was performed of rectal cancer patients treated in 2000–2013 from the National Oncology Data Alliance™ (NODA), a pooled database of cancer registries from >150 US hospitals. The NODA contains the same data submitted to state cancer registries and SEER combined with validated radiotherapy and chemotherapy records. Eligible patients were treated with neoadjuvant CRT followed by proctectomy and had complete data on treatment start dates, radiotherapy dose, clinical tumor (cT) and ypT stage, and number of positive nodes at surgery (n = 3298 patients). Multivariable logistic regression was used to assess the predictive value of independent variables on achieving a pCR.
Results: On multivariable regression, radiotherapy dose, cT stage, and time interval between CRT and surgery were significant predictors of achieving a pCR. After adjusting for the effect of other variates, patients treated with higher radiotherapy doses were also more likely to have negative nodes at surgery and be downstaged from cT3-T4 and/or node positive disease to ypT0-T2N0 after neoadjuvant CRT.
Conclusion: Our study suggests that increasing dose significantly improved pCR rates and downstaging in rectal cancer patients treated with neoadjuvant CRT followed by surgery.
Neoadjuvant chemoradiation therapy (CRT) followed by total mesorectal excision (TME) decreases local recurrence and acute and late toxicity for patients with T3-T4 and/or node positive rectal cancer compared to TME and adjuvant CRT [Citation1–2]. The degree of treatment response is variable, however, with some patients achieving a pathologic complete response (pCR) compared to limited or no response. The addition of concurrent fluorouracil and leucovorin to radiotherapy improves both pCR rates and local control [Citation3,Citation4]. Five clinical trials have reported pCR rates after neoadjuvant CRT with and without the addition of oxaliplatin, and four showed no difference in tumor response [Citation5–9].
Dose-escalated CRT may improve tumor response rates, but the degree of pathologic response observed with varying dose has not been well characterized in this population. The purpose of this study is to assess the impact of radiation dose on post-therapy pathologic tumor (ypT) and nodal (ypN) stage in rectal cancer patients treated with neoadjuvant CRT followed by surgery and to quantify the effect of other prognostic factors.
Material and methods
Patient selection
De-identified data on all patients diagnosed with rectal adenocarcinoma in 2000–2013 were extracted from the National Oncology Data Alliance™ (NODA). The NODA is a pooled database that captures all newly diagnosed cancer cases at >150 American College of Surgeons (ACOS) Commission on Cancer accredited hospitals that use the same registry software (Elekta/IMPAC Medical Systems, Inc., Sunnyvale, CA, USA). The NODA contains exactly the same data submitted to state tumor registries and to Surveillance, Epidemiology, and End Results (SEER), in regions that participate in SEER. In addition, it also provides records of the chemotherapy and radiotherapy administered, which are manually verified by trained staff.
The study population was compiled from all patients with T3-T4 and/or node positive rectal cancer treated with neoadjuvant CRT followed by mesorectal excision with complete data on radiotherapy dose, clinical tumor (cT) stage, ypT stage, number of lymph nodes examined, number of positive lymph nodes at surgery, and the treatment start dates with chemotherapy, radiotherapy and surgery. All patient identifiers were removed from the data set. Neoadjuvant CRT was defined as chemotherapy starting within seven days of the start of radiotherapy. In order to minimize the effect of differential fractionation regimens, only patients treated at 1.8 Gy per fraction were included. Dose was analyzed as a categorical variable with patients divided into four groups. Patients treated with <45 Gy (defined as 36–43.2 Gy), 45 Gy, and 50.4 Gy were compared to patients receiving 54 Gy as the referent category. Patients who underwent short-course neoadjuvant radiotherapy, had metastatic disease at diagnosis, or had history of prior malignancy were excluded. The final study population contained 3298 evaluable patients.
Patient characteristics included age, sex, race, marital status, cT stage, clinical nodal (cN) stage, grade, ypT stage, ypN stage, number of lymph nodes examined, circumferential resection margin (CRM) status, year of diagnosis, year of treatment, concurrent chemotherapy regimen, radiotherapy dose, type of surgery [abdominoperineal resection (APR) versus low anterior resection (LAR)], academic versus community hospital type, metropolitan versus non-metropolitan hospital site, medical comorbidities, and time interval between the completion of CRT and surgery. This study was conducted with the approval of the City of Hope Institutional Review Board.
The primary objective was to determine if increasing radiotherapy dose was associated with pCR (defined as ypT0N0) after trimodality therapy. The secondary aims were to identify factors associated with finding negative lymph nodes (ypTxN0) at surgery and downstaging from cT3-T4 and/or node positive disease to ypT0-T2N0 and to correlate ypT and ypN status in a large series of patients treated with neoadjuvant CRT followed by proctectomy.
Statistical analysis
Statistical analysis was performed using SPSS v. 18.0 (SPSS Inc., Chicago). Differences between categorical variables were examined with the χ2-test for proportions, and continuous variables were compared by analysis of variance (ANOVA). Multivariable logistic regression using a backward elimination model for all covariates was employed to identify factors associated with achieving a pCR, ypTxN0 status, and downstaging from cT3-T4 and/or node positive disease to ypT0-T2N0 after neoadjuvant CRT. The threshold for keeping a variable in the model with backward elimination was p < .05. Time interval between the end of CRT and surgery was analyzed as a continuous variable, with a maximum delay of 20 weeks. The association between ypT and ypN status was examined by the Jonckheere-Terpstra test. Survival curves were plotted by Kaplan-Meier and compared using the log-rank test.
Missing data on race (1.5%), marital status (7.7%), cN stage (5.5%), grade (13.9%), type of surgery (9.5%), surgical margin (2.2%), and concurrent chemotherapy (12.0%) were handled by multiple imputation using chained equations [Citation10]. The imputation models included the same variables as analysis models and five imputed datasets were created. Multivariable outcomes for achieving pCR, ypTxN0, and downstaging with and without imputation produced virtually identical parameter estimates and p-values; therefore results are presented only for the total dataset with missing values replaced by imputation.
Results
Patient characteristics
Patient demographics and tumor characteristics are presented in . Median age was 61 years [interquartile range (IQR) 52–69 years]. The clinical characteristics of patients stratified by radiotherapy dose are also displayed. The median number of lymph nodes examined was 11 (IQR 6–16 lymph nodes). Median follow-up was 4.3 years (IQR 2.2–6.9 years) by Kaplan-Meier estimated potential follow-up. At the close-out date, 25% of patients had died.
Table 1. Patient demographics and tumor characteristics.
Multivariable logistic regression and analysis of factors associated with tumor response
On multivariable regression, cT stage, time interval between the completion of neoadjuvant CRT and surgery and radiotherapy dose were significant predictors of achieving pCR (). Concurrent chemotherapy regimen, cN stage, grade and other factors were not significant. In aggregate, 14.9% of patients were found to have pCR in this series. Rates of pCR increased with lower cT stage and with longer time intervals between the end of CRT and surgery. The observed pCR rates increased sequentially with increasing dose, from 10.9% at 45 Gy, 15.9% at 50.4 Gy, and 18.8% at 54 Gy. Adjusting for the effect of other variates, increasing radiotherapy dose was significantly associated with the probability of achieving a pCR.
Table 2. Odds ratios for tumor response.
On multivariable regression, cN stage, time interval between the end of neoadjuvant CRT and surgery, and radiotherapy dose were significantly associated with finding negative lymph nodes at surgery (). cT stage and other factors were not significant. The percentage of patients found to have negative lymph nodes at surgery increased with higher radiotherapy doses, reaching 65.7% at 45 Gy, 69.1% at 50.4 Gy, and 73.4% at 54 Gy.
Patients treated with higher radiotherapy doses were more likely to be downstaged from cT3-T4 and/or node positive disease to ypT0-T2N0 after neoadjuvant CRT. The observed percentage of patients who were downstaged increased from 36.7% with 45 Gy, 43.7% with 50.4 Gy, and 49.4% with 54 Gy. On multivariable logistic regression, cT stage, cN stage, time interval between the end of neoadjuvant CRT and surgery, and radiotherapy dose were significant predictors of downstaging to ypT0-T2N0; all other variates were not significant ().
Table 3. Odds ratios for tumor response.
Patients treated with higher radiotherapy doses were also less likely to have a positive CRM, decreasing from 9.9% at <45 Gy, to 6.3% at 45 Gy, 5.0% at 50.4 Gy, and 4.4% at 54 Gy (p < .01).
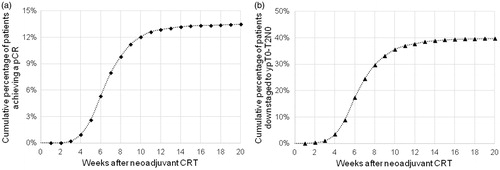
The cumulative proportion of patients who achieved a pCR was plotted as a function of the number of weeks elapsed after the completion of neoadjuvant CRT, as shown in . The cumulative proportion of patients who were downstaged to ypT0-T2N0 by time after the completion of neoadjuvant CRT is shown in . The percentage of patients who achieved a pCR and were downstaged increased most rapidly between four and eight weeks, and the rate of change leveled off after 10–12 weeks.
Figure 1. (a) Cumulative proportion of patients who achieved a pathologic complete response (pCR) by the time elapsed after the completion of neoadjuvant chemoradiation therapy (CRT). (b) Cumulative proportion of patients who were downstaged from cT3-T4 and/or node positive disease to ypT0-T2N0 by the time elapsed after the completion of neoadjuvant CRT.
Survival outcomes and the association between ypT and ypN stage
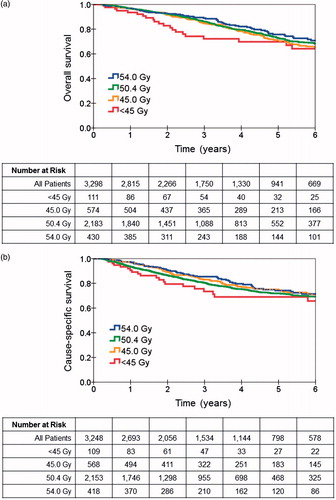
Five-year overall survival for the entire cohort was 70.2% and the median survival was 9.7 years (95% CI 8.9–10.5 years). The Kaplan-Meier curves demonstrated no significant difference in overall survival in patients treated with different radiotherapy doses (p = .27), as shown in . This pattern was also observed for cause-specific survival (p = .30), as shown in .
Figure 2. (a) Overall survival and (b) cause-specific survival by radiotherapy dose. The Kaplan-Meier curves showed no significant difference in overall survival (p = .27) or cause-specific survival (p = .30) in patients treated with different radiotherapy doses.
Patients were categorized into three groups on the basis of their ypT and ypN stage after trimodality therapy: ypT0N0 as pCR, ypT1-2N0 as intermediate response, and ypT3-4N0 or ypTxN + as poor response. The five-year cause-specific survival was 89.1% for patients who achieved pCR, 84.5% for patients with intermediate response, and 62.3% for patients with a poor response (p < .001). The difference in cause-specific survival for patients with pCR and intermediate response was also statistically significant (p = .03).
After neoadjuvant CRT, 2249 of 3298 patients (68.2%) were found to have negative lymph nodes at surgery in this series. The presence of pathologically involved lymph nodes was significantly correlated with ypT stage (Jonckheere-Terpstra test, p < .001). Lymph node involvement as a function of ypT stage is shown in . Patients with ypT0 tumors at surgery were found to have positive nodes in 10.6% of cases, increasing to 13.2% in ypTis-T1, >20% in ypT2, and >40% in ypT3-T4 patients.
Table 4. Nodal involvement after neoadjuvant CRT by ypT stage.
Discussion
The results of this study suggest that patients treated with higher radiotherapy doses during neoadjuvant CRT were significantly more likely to achieve pCR and have negative lymph nodes at surgery compared to patients who received lower doses. Higher radiotherapy doses were also associated with a small but statistically significant reduction in positive CRMs. To our knowledge, this is the largest series to suggest that increasing radiotherapy doses are associated with tumor response at mesorectal excision.
Pretreatment tumor burden and time interval between CRT and surgery have been previously identified as important predictors of treatment response [Citation11]. Compared with these factors, the role of increasing dose on tumor response has been poorly characterized in the setting of concurrent chemotherapy. In RTOG 0012, Mohiuddin et al. observed high pCR rates of 26–30% in patients treated with higher radiation doses compared to historical controls [Citation12]. Wiltshire et al. reported a trend toward increased pCR rates after neoadjuvant CRT with higher doses in three consecutive phase II studies, rising from 15% at 40 Gy in 20 fractions, to 23% at 46 Gy in 23 fractions, and 33% at 50 Gy in 25 fractions (p = .07) [Citation13]. In the ACCORD 12 trial, a significant increase in complete or near complete response was observed with the neoadjuvant regimen of 50 Gy plus capecitabine/oxaliplatin compared to 45 Gy plus capecitabine, but the difference in pCR rates of 19.2% versus 13.9% did not reach statistical significance (p = .09). Given that no improvement in pCR rates was observed with the addition of oxaliplatin to neoadjuvant CRT in the STAR-01, NSABP R-04, and PETACC-6 trials [Citation6–8], this suggests that radiation dose escalation was responsible for the improvement in response rates observed in ACCORD 12 [Citation5].
cT stage is an important predictive factor for tumor response and likely contributes to the observed variations in the pCR rates in published series. Garcia-Aguilar et al. reported a 44% pCR rate in cT2N0 patients treated with neoadjuvant CRT with capecitabine and oxaliplatin followed by local excision on a phase II trial [Citation14]. In a series of cT2-T4N0-N2 rectal cancer patients treated with CRT to 50.4–54 Gy, Habr-Gama et al. reported clinical complete response (cCR) rates in 49% (90 of 183) of treated patients, who were then managed with a watch and wait strategy [Citation15]. In comparison, in a study by Maas et al., only 11% (21 of 192) achieved cCR after CRT with 50.4 Gy followed by adjuvant chemotherapy and could be managed with this watch and wait strategy [Citation16]. The wide range of complete response rates observed in published series, ranging from 44% in cT2N0 patients compared to <20% in other studies, support the notion that cT stage and patient selection have a significant effect on pCR rates [Citation14–16]. Due to inevitable differences between the patients included in retrospective series, comparison between tumor response rates in the literature remains problematic.
The dependency of tumor response on the time interval between neoadjuvant CRT and surgery has also been previously established [Citation17,Citation18]. Francois et al. reported that patients treated with neoadjuvant radiotherapy followed by surgery 6–8 weeks later achieved increased tumor downstaging and pCR rates compared to those having surgery within two weeks [Citation19]. Pettersson et al. demonstrated that longer delays between short-course radiotherapy and surgery up to 4–8 weeks resulted in higher pCR rates than patients taken to surgery within one week (10.1% vs. 1.7%) [Citation20]. Lengthening the interval between CRT and surgery, however, could increase risk by delaying adjuvant chemotherapy and increasing fibrosis and post-operative complications, and a 6–8-week delay remains routine practice after long-course CRT. Garcia-Aguilar et al. showed that pCR rates increased sequentially in patients treated with neoadjuvant CRT and surgery performed 6–8 weeks later or after two, four or six cycles of mFOLFOX6 without an increase in surgical difficulty or complications [Citation21].
In a large patient cohort, our study showed that the utilization of increasing radiotherapy doses during neoadjuvant CRT was significantly associated with higher pCR rates at mesorectal excision. A similar monotonic dose response was also observed for the probability of finding negative lymph nodes at surgery and for downstaging from cT3-T4 and/or node positive disease to ypT0-T2N0. Although retrospective series from tertiary care centers may benefit from more standardized surgical and pathologic procedures, sample size may limit their ability to detect smaller effect sizes due to reduced statistical power. Larger sample size is an advantage of pooled registries and population-based databases.
Our study is retrospective and has important limitations. Pathologic tumor response was determined by pathology reports coded into a merged cancer registry, not by a centralized pathology review. As a result, it is not possible to analyze or report tumor regression grading (TRG), percentage of residual tumor cells or other related metrics. Although this study is the largest to address pCR rates in rectal cancer patients treated with neoadjuvant CRT followed by mesorectal excision, information on certain important variables, including surgical technique, use of and adherence to the prescribed chemotherapy, and post-operative complications, were not available. In addition, the outcome measures were limited to tumor response, overall survival, and cause-specific survival. Data regarding toxicity, post-treatment bowel function, and local recurrence cannot be extracted. Pooled cancer registries do not capture a statistically representative portion of the national cancer population, and certain patient groups may be underrepresented. Patient selection can influence the results in population-based observational series and must be considered when applying our results to clinical practice. For example, patients who received lower radiotherapy doses, and the group treated with <45 Gy in particular, were more likely to discontinue treatment early due to toxicity, progression, or for other unfavorable factors. This may have contributed to inferior response rates.
Data completeness, enabled by use of a stringent database search algorithm and fastidious inclusion criteria, helps to counter the likelihood that the dose response is due to selection bias. The analysis accounted for many known variables that can influence tumor response, including chemotherapy, clinical and pathologic staging, and time interval between CRT and surgery. In addition, patient characteristics and reported medical comorbidities in the subgroup of patients treated with higher radiotherapy doses were similar to, or even favored, the patients who received lower doses (). We acknowledge that observational studies cannot replace randomized data as the standard for outcomes research. Population-based analyses, however, can provide measures of the effectiveness of therapies in the general population and address important clinical questions that have not been tested in prospective trials.
TRG is an important metric and one that could not be explored in this dataset. However, inter-observer standardization and limitations in generalizability of this measure of tumor response have led to alternative definitions of tumor regression [Citation22]. In contrast, ypT and ypN staging are easily identified, are routinely reported in both pathology reports and in cancer registries, and are correlated with oncologic outcomes. As a result, they are also a more practical measure of treatment response than TRG [Citation23,Citation24].
The identification of patients that may be candidates for transanal excision or non-operative management based on objective tumor response criteria, especially in less than optimal surgical candidates, is of great interest. Better selection based on pretreatment characteristics and the administration of appropriate therapy to maximize tumor response is essential to advance these conservative treatment approaches. A recent meta-analysis identified a pooled pCR rate of 20.4% (95% CI 16.8–24.5%) in locally advanced rectal cancer patients treated with neoadjuvant therapy to doses of ≥60 Gy [Citation25]. Modest dose escalation has been used with encouraging results in patients considered for conservative management [Citation17–19]. Appelt et al. reported encouraging cCR rates and freedom from salvage APR with dose-escalated CRT and endorectal brachytherapy boost in a prospective series of T2-T3N0-N1 patients with tumors located <6 cm from the anal verge [Citation26].
In our study, 10.6% of patients with ypT0 tumors at mesorectal excision were found to have positive lymph nodes. This suggests that some patients with complete response in the primary tumor after neoadjuvant therapy will still have unaddressed nodal disease if managed with transanal excision or a watch and wait strategy. Accurate assessment of clinical response and post-treatment diagnostic imaging are critical to better identify patients who are less likely to respond to neoadjuvant therapy and to correctly identify patients that can be managed conservatively [Citation27]. Although it is important to note that clinicopathological concordance between cCR and pCR is reported to be modest [Citation28], it is reasonable to infer that treatment strategies that increase the probability of achieving pCR will also favorably influence cCR rates. Our results indicate that radiotherapy dose is an important parameter that should not be overlooked in rectal cancer patients treated with neoadjuvant CRT. Higher radiation doses should be considered in patients treated with neoadjuvant CRT and particularly in those planned for transanal excision or non-operative management. Dose escalation could be further evaluated using advanced radiotherapy techniques to limit toxicity such as dose-painting intensity modulated radiotherapy, or with the addition of endorectal brachytherapy.
Conclusions
In a large population-based cohort, our analysis suggests that rectal cancer patients treated with higher radiotherapy doses were more likely to achieve a pCR and have negative nodes at mesorectal excision after neoadjuvant CRT. In addition, ypT stage was positively correlated with nodal involvement at surgery. In this series, 10–15% of ypT0-T1 patients were found to have positive nodes at surgery. In our practice, we routinely recommend treatment with radiotherapy doses up to 50.4–54 Gy. The effect of radiation dose escalation should be tested in future randomized trials.
Disclosure statement
None to declare.
References
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40.
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693–701.
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620–5.
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23.
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638–44.
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773–80.
- O'Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol 2014;32:1927–34.
- Schmoll HJ, Haustermans K, Rice TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: disease-free survival at interim analysis. J Clin Oncol 2014;32:5s (suppl; abstr 3051).
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomized, phase 3 trial. Lancet Oncol 2015;16:979–89.
- van der Heijden GJ, Donders AR, Stijnen T, et al. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol 2006;59:1102–9.
- Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 2011;29:3163–72.
- Mohiuddin M, Paulus R, Mitchell E, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys 2013;86:523–8.
- Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys 2006;64:709–16.
- Garcia-Aguilar J, Shi Q, Thomas CR, Jr., et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 2012;19:384–91.
- Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822–8.
- Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633–40.
- Habr-Gama A, Perez RO, Proscurshim I, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys 2008;71:1181–8.
- Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 2004;47:279–86.
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396–402.
- Pettersson D, Lörinc E, Holm T, et al. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg 2015;102:972–8.
- Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957–66.
- Demetter P, Vandendael T, Sempoux C, et al. Need for objective and reproducible criteria in histopathological assessment of total mesorectal excision specimens: lessons from a national improvement project. Colorectal Dis 2013;15:1351–8.
- Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770–6.
- Hall MD, Schultheiss TE, Smith DD, et al. Impact of total lymph node count on staging and survival after neoadjuvant chemoradiation therapy for rectal cancer. Ann Surg Oncol 2015;22:580–7.
- Burbach JP, den Harder AM, Intven M, et al. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol 2014;113:1–9.
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015;16:919–27.
- Perez RO, Habr-Gama A, São Julião GP, et al. Optimal timing for assessment of tumor response to neoadjuvant chemoradiation in patients with rectal cancer: do all patients benefit from waiting longer than 6 weeks? Int J Radiat Oncol Biol Phys 2012;84:1159–65.
- Smith FM, Chang KH, Sheahan K, et al. The surgical significance of residual mucosal abnormalities in rectal cancer following neoadjuvant chemoradiotherapy. Br J Surg 2012;99:993–1001.
