Abstract
Background: The St Gallen surrogate definition of the intrinsic subtypes of breast cancer consist of five subgroups based on estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor type 2 (HER2), and Ki-67. PgR and Ki-67 are used for discriminating between the ‘Luminal A-like’ and ‘Luminal B-like (HER2-negative)’ subtypes. Histological grade (G) has prognostic value in breast cancer; however, its relationship to the St Gallen subtypes is not clear. Based on a previous pilot study, we hypothesized that G could be a primary discriminator for ER-positive/HER2-negative breast cancers that were G1 or G3, whereas Ki-67 and PgR could provide additional prognostic information specifically for patients with G2 tumors. To test this hypothesis, a larger patient cohort was examined.
Patients and methods: Six hundred seventy-one patients (≥35 years of age, pT1-2, pN0-1) with ER-positive/HER2-negative breast cancer and complete data for PgR, Ki-67, G, lymph node status, tumor size, age, and distant disease-free survival (DDFS; median follow-up 9.2 years) were included.
Results: ‘Luminal A-like’ tumors were mostly G1 or G2 (90%) whereas ‘Luminal B-like’ tumors were mostly G2 or G3 (87%) and corresponded with good and poor DDFS, respectively. In ‘Luminal B-like’ tumors that were G1 (n = 23), no metastasis occurred, whereas 14 of 40 ‘Luminal A-like’ tumors that were G3 metastasized. In the G2 subgroup, low PgR and high Ki-67 were associated with an increased risk of distant metastases, hazard ratio (HR) and 95% confidence interval (CI) 1.8 (0.95–3.4) and 1.5 (0.80–2.8), respectively.
Conclusions: Patients with ER-positive/HER2-negative/G1 breast cancer have a good prognosis, similar to that of ‘Luminal A-like’, while those with ER-positive/HER2-negative/G3 breast cancer have a worse prognosis, similar to that of ‘Luminal B-like’, when assessed independently of PgR and Ki-67. Therapy decisions based on Ki-67 and PgR might thus be restricted to the subgroup G2.
Adjuvant systemic therapy has improved survival among breast cancer patients, the majority of which have estrogen receptor (ER)-positive, human epidermal growth factor receptor type 2 (HER2)-negative disease. For patients with this subtype, adjuvant endocrine therapy is usually recommended, often in combination with chemotherapy. One of the greatest challenges within this group of patients is to identify those with good prognosis for whom chemotherapy can be avoided [Citation1]. In 2013, the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer updated their surrogate panel, based on ER, Ki-67, progesterone receptor (PgR), and HER2, for classification of the intrinsic subtypes of breast cancer [Citation2]. In this update, ER-positive/HER2-negative breast cancer was further divided into ‘Luminal A-like’ and ‘Luminal B-like (HER2 negative)’ subgroups wherein the prognosis of patients with the former is better than that for the latter. In the ‘Luminal A-like’ group, adjuvant chemotherapy might thus be avoided in the absence of other negative prognostic factors.
Histological grade (G) has repeatedly been shown to be a strong and independent prognostic factor [Citation3–6], however, in 2013 the majority of St Gallen expert panelists voted that G3 could not be used as a substitute for high Ki-67 [Citation2]. In contrast, in a pilot study that investigated the role of G in breast cancer prognosis in addition to that afforded by the 2013 St Gallen classification system we found that in 161 premenopausal lymph node-negative patients with ER-positive/HER2-negative breast cancer, G was strongly associated with St Gallen subtypes [Citation7]. Indeed, ‘Luminal A-like’ were mostly G1 or G2, whereas ‘Luminal B-like’ were usually G2 or G3 [Citation7]. Of the cases that diverged, a follow-up period of 10 years revealed that two out of four patients with ‘Luminal A-like’ G3 breast cancer developed distant metastases and had a prognosis more similar to that of ‘Luminal B-like’ breast cancer, whereas of the three patients with ‘Luminal B-like’ breast cancer that were G1, not one experienced relapse and thus their clinical outcome was more similar to that of ‘Luminal A-like’ breast cancer. These results, although based on a small number of cases, suggest that, independent of PgR and Ki-67, patients with ER-positive/HER2-negative breast cancers that are G1 might have a better prognosis than those with G3.
The primary aim of the present investigation was to use an independent patient material to confirm the additional prognostic value of G to that of the 2013 St Gallen surrogate classification of ER-positive/HER2-negative breast cancer. We hypothesized that for the ER-positive/HER2-negative subgroup of patients, G would be the first discriminator for those with G1 or G3 tumors, whereas Ki-67 and PgR would provide additional prognostic information specifically for patients with G2 tumors. As a secondary aim, the prognostic importance of PgR and Ki-67 was evaluated in patients with G2, ER-positive/HER2-negative breast cancer.
Patients and methods
Patients
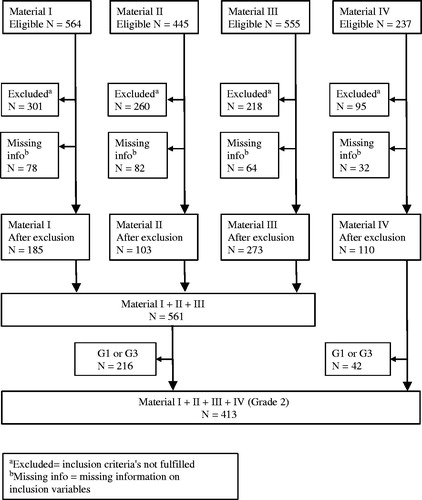
For the primary aim, we included breast cancer patients from two randomized multicenter trials (Patient materials I and II) and one additional cohort (Patient material III) (). Patients with complete information regarding follow-up, number of positive lymph nodes, tumor size, ER, PgR, HER2, Ki-67, and G were included (). Patients with at least one of the following characteristics were excluded: ER negativity, HER2 positivity, <35 years of age, ≥4 positive lymph nodes, tumor size >50 mm. Patients with these characteristics are most likely candidates for adjuvant chemotherapy without consideration of other prognostic factors.
Table 1. Patient and tumor characteristics.
For the second aim, an additional 110 patients with G2 tumors were included (Patient material IV; see below). These patients were not included when addressing the primary aim as they were part of the pilot study [Citation7].
Patient material I: (N = 185). Premenopausal patients with stage II breast cancer participated in a randomized trial comparing the effect of two years of tamoxifen treatment versus no adjuvant systemic treatment. The original trial included 564 patients enrolled in the South and South-East Swedish Health Care Regions between 1986 and 1991 [Citation8].
Patient material II: (N = 103). Postmenopausal patients with stage II breast cancer were enrolled, between 1983 and 1991, in a randomized trial launched by the Swedish Breast Cancer group of two versus five years of adjuvant tamoxifen treatment (Swedish Breast Cancer Cooperative Group 1996) [Citation9]. From the original trial, paraffin-embedded tumor material was collected from a subgroup of patients treated with tamoxifen for two years in the South Swedish Health Care Region, for comparison of cytosol and immunohistochemistry methods for the analyses of ER and PgR [Citation10]. This subgroup was included in the present study.
Patient material III: Bone marrow metastases cohort (N = 273). The purpose of the original cohort was to study the prognostic importance of the presence of cytokeratin-positive cells in the bone marrow. It included 555 patients recruited from three hospitals in the South Swedish Health Care Region between 1999 and 2003 [Citation11].
Patient material IV: SB91b (N = 110). Premenopausal, lymph node-negative women were enrolled between 1991 and 1994 in a trial administrated by the South Swedish Breast Cancer Group, for evaluation of the prognostic importance of prospectively analyzed S-phase fraction by flow cytometry [Citation12]. The original trial included 237 patients of which 110 patients with G2 tumors were included in the present study.
Evaluation of histological grade
Histological grade of whole tissue sections was reevaluated by breast pathologists according to Elston and Ellis [Citation3], as previously described for patient materials I–III. Patient material IV was reevaluated by one of the authors of the present study (CWE) using the same guidelines.
Analysis of ER, PgR, Ki-67, and HER2
The expression levels of ER, PgR, Ki-67, and HER2 were evaluated on whole sections or tissue microarrays as previously described [Citation8,Citation13,Citation14]. Two core biopsies were evaluated from each formalin-fixed, paraffin-embedded breast cancer tissue, and the one with the highest percentage of positively stained cells was chosen. All cores were 0.6 mm in diameter with the exception of those used for ER and PgR analyses in Patient material IV that were 1.0 mm in diameter.
Cutoffs: ER and PgR positivity were defined as >10% stained nuclei, high Ki-67 as >20% stained nuclei, and HER2 positivity as 3 + or amplified 2+. It should be mentioned that since ER and PgR had previously been analyzed and reported in categories (positive vs. negative), we could not strictly apply the cutoffs according to the St Gallen recommendations (ER positivity: ≥1% and high PgR: ≥20%). Based on our experience from one of the included cohorts (SB91b), however, only a very small percentage of the tumors would have been influenced by this difference.
The 2013 St Gallen classification of intrinsic subtypes
St Gallen classification, based on ER, PgR, Ki-67, and HER2, was used to divide ER-positive/HER2-negative breast cancer cases into two intrinsic subtypes, as follows:
‘Luminal A-like’: ER-positive, PgR-positive, HER2-negative, and low Ki-67;
‘Luminal B-like (HER2-negative)’: ER-positive, HER2-negative, and one or both of high Ki-67 and PgR-negative.
Statistics
Distant disease-free survival (DDFS) was chosen as the endpoint in the present study. Differences in DDFS between subgroups of patients were evaluated using Kaplan-Meier estimates and log-rank tests. All tests were stratified for patient material. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were estimated using Cox regression, also stratified for patient material. Owing to violations of proportional hazards assumptions for most variables included in the models, the follow-up was restricted to the first 10 years after diagnosis. This action led to fewer problems with non-proportional effects, but all effects should nevertheless be interpreted as average effects over time and not as constant effect estimates valid independent of follow-up time. All analyses were carried out using Stata version 14 (StataCorp LP, College Station, TX, USA, 2015).
Results
Histological grade in ‘Luminal A-like’ and ‘Luminal B-like (HER2-negative)’ breast cancer
The 2013 St Gallen International Panel of Experts guidelines were used to classify breast cancers from patient materials I–III. According to these guidelines, 390 (70%) of the 561 ER-positive/HER2-negative tumors were classified as ‘Luminal A-like’ whereas the remaining 171 (30%) were ‘Luminal B-like’ (). In terms of prognosis, after a median follow-up of 9.3 years for patients alive and free from distant metastases, the latter subgroup had significantly worse DDFS compared with the former (HR = 1.5, 95% CI 1.0–2.3; ). The distribution of G in these two subgroups was also reviewed. The majority of ‘Luminal A-like’ tumors were either G1 or G2 (350/390; 90%), whereas a high proportion of ‘Luminal B-like’ tumors were G2 or G3 (148/171; 87%; ). Notably, among the 40 patients with ‘Luminal A-like’ tumors that were G3, 14 (35%) developed distant metastases during the follow-up period. In contrast, of the 23 patients with ‘Luminal B-like’ breast cancers that were G1, none developed distant metastases during follow-up (median follow-up for these 23 patients: 9.4 years, range: 5.5–10 years). The prognostic importance of G3 in ‘Luminal A-like’ and G1 in ‘Luminal B-like’ breast cancer is further illustrated in . As most patients with ER-positive/HER2-negative breast cancer are treated with adjuvant endocrine therapy, the prognostic value of St Gallen classification was examined in endocrine-treated patients separately (). Similar to the results above, DDFS was worse for patients with ‘Luminal B-like’ compared with that for those with ‘Luminal A-like’ breast cancers (HR = 1.6, 95% CI 0.98–2.7). Similarly, when G was also accounted for, the prognostic importance of G3 in ‘Luminal A-like’ and G1 in ‘Luminal B-like (HER2-negative)’ breast cancer as indicators of poor and good prognosis, respectively, was also confirmed in this subgroup of patients ().
Figure 2. Distant disease-free survival (DDFS) by St Gallen subtypes, ‘Luminal A-like’ and ‘Luminal B-like (HER2-negative)’, for all patients (a) and for patients treated with adjuvant endocrine therapy alone (b).
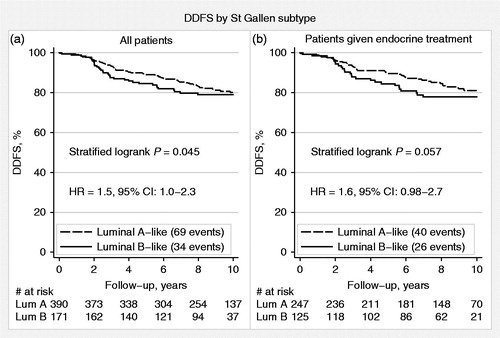
Figure 3. Distant disease-free survival (DDFS) by histological grade (G) and St Gallen subtypes, ‘Luminal A-like’ and ‘Luminal B-like (HER2-negative)’, for all patients (a) and for patients treated with adjuvant endocrine therapy alone (b).
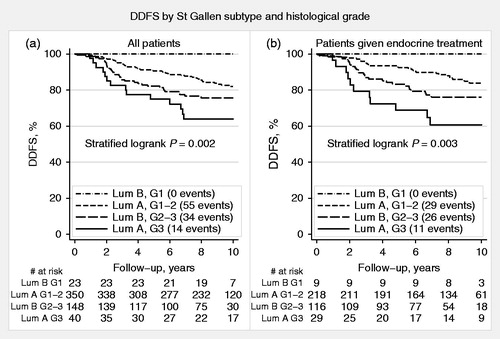
Table 2. Patient and tumor characteristics of ‘luminal A-like’ versus ‘luminal B-like (HER2-negative)’.
To further assess prognostic factors in our study cohort, multivariable analysis was performed including G, St Gallen subtypes, tumor size, lymph node status, and patient age. Among these, only G and lymph node status were found to be significant prognostic factors (). Similar results were obtained when patients treated with adjuvant endocrine therapy were analyzed separately ().
Table 3. Multivariable analysis of all patients (N = 561; stratified for patient material).
Table 4. Multivariable analysis of patients treated with endocrine therapy (N = 372; stratified for patient material).
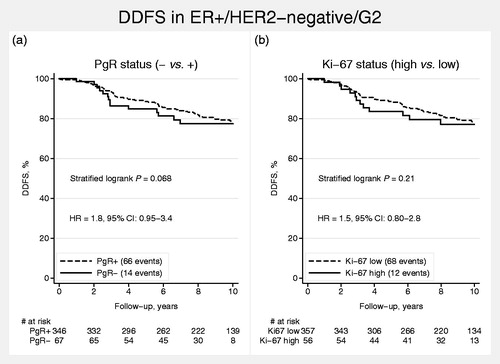
PgR and Ki-67 in G2 breast cancer
As G2 was not clearly associated with prognosis of either ‘Luminal A-like’ or ‘Luminal B-like’ breast cancer, PgR and Ki-67 were evaluated as possible prognostic discriminators in G2 tumors. Although both PgR negativity and high Ki-67 were associated with poor prognosis in G2 tumors, univariable analyses showed weak evidence for prognostic discrimination [PgR (negative vs. positive): HR = 1.8, 95% CI 0.95–3.4; Ki-67 (high vs. low): HR = 1.5, 95% CI 0.80–2.8; ].
Discussion
In the present study, histological grade (G) added prognostic information to that obtained using the 2013 St Gallen surrogate definition for the intrinsic subtypes of breast cancer. Our findings confirm that breast cancers designated ER-positive/HER2-negative that are G1 represent a good prognosis group, with a prognosis similar to that of ‘Luminal A-like’ breast cancer. In contrast, ER-positive/HER2-negative breast cancers that are G3 have worse prognosis, similar to that of ‘Luminal B-like’ breast cancer. Notably, this could be ascertained independent of Ki-67 and PgR. Moreover, these findings were essentially unchanged when the effects of G and St Gallen classification on prognosis were assessed in patients treated with adjuvant endocrine therapy alone. This therapy is generally recommended for patients with ER-positive/HER2-negative breast cancer, alone or as chemo-endocrine therapy. Based on our findings, the importance of Ki-67 and PgR could be restricted to G2 breast cancers for the discrimination between good and poor prognosis in ER-positive/HER2-negative breast cancer.
In our study, 10% of ‘Luminal A-like’ were G3 and 13% of ‘Luminal B-like’ were G1. A recent publication by Maisonneuve and coworkers obtained comparative figures of 2.5% and 4.6%, respectively [Citation15] as did Engstrøm and colleagues, who reported 10.3% G3 in ‘Luminal A-like’ and 8.0% G1 in ‘Luminal B-like’ in a study of 682 patients with ER-positive/HER2-negative breast cancer [Citation16]. The occurrence of poorly differentiated luminal A tumors (14.1%) as well as well differentiated luminal B tumors (9.4%) has also been demonstrated in a study based on the PAM50 gene set [Citation17]. Although accounting for a small percentage of cases, because G3 in ‘Luminal A-like’ and G1 in ‘Luminal B-like’ inverted the expected prognosis dictated by the St Gallen subtypes alone, these findings could critically influence disease treatment for patients of these subgroups.
Similar to our study, Maisonneuve and colleagues suggested that G could be incorporated as a first discriminator for ER-positive/HER2-negative breast cancer, where G1 was a strong indicator for the ‘Luminal A-like’ subtype and G3 for the ‘Luminal B-like’. The main focus of their study was, however, to evaluate the prognostic importance of PgR and its relation to Ki-67 in the ER-positive/HER2-negative breast cancer subgroup. Both Ki-67 and PgR have been reported to be of prognostic importance for ER-positive disease in several studies [Citation18,Citation19]. Indeed, based on the study of Prat and colleagues [Citation20], PgR was introduced into the St Gallen breast cancer subtype definition in 2013. Maisonneuve and coworkers showed that the prognostic importance of PgR was restricted to the intermediate Ki-67 subgroup (14–20%), and that it did not provide any additional prognostic information for the subgroups with either low (<14%) or high (≥20%) Ki-67. Our study, which was focused on G as the initial watershed, showed weak evidence for Ki-67 as a prognostic factor in patients with G2 tumors (p = 0.22), and the evidence for PgR was only slightly stronger (p = 0.08). The weak evidence may, however, to a large extent be a power problem. For PgR, this pattern remained whether patients treated with adjuvant endocrine therapy were studied separately (data not shown).
One drawback with G, however, is its limited inter-observer reproducibility [Citation21,Citation22]. In spite of this, it has repeatedly been shown to be a strong prognostic factor [Citation3–6]. Furthermore, it is cheap and easily evaluated routinely in the clinical setting. Also, by using strict guidelines, the concordance between different evaluators can be improved [Citation23]. In this context it should also be mentioned that limited inter-observer reproducibility is also a well known problem for Ki-67 [Citation24,Citation25].
In conclusion, our findings suggest that patients above or equal to the age of 35 years at diagnosis with T1-2, N0-1, ER-positive/HER2-negative/G1 breast cancer have a prognosis similar to that of ‘Luminal A-like’, without consideration of Ki-67 and PgR. For this group of patients, chemotherapy might be avoided in the absence of other adverse prognostic factors. In contrast, patients with ER-positive/HER2-negative/G3 breast cancer have a worse prognosis, similar to that of ‘Luminal B-like’. Therapy decisions based on Ki-67 and PgR might thus be restricted to the ER-positive/HER2-negative/G2 subgroup of breast cancers.
Acknowledgments
We are indebted to participating departments of the South Swedish Breast Cancer Group and South East Swedish Breast Cancer Group for providing samples and clinical follow-up.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
The study was supported by funds from the Swedish Cancer Society, the Gunnar Nilsson Cancer Foundation, the Mrs. Berta Kamprad Foundation, the Anna and Edwin Bergers Foundation, the Swedish Breast Cancer Association (BRO), the Swedish Cancer and Allergy Foundation, The Scientific Committee of Blekinge County Council, Skåne County Council’s Research and Development Foundation, and Governmental Funding of Clinical Research within the National Health Service.
References
- Dowsett M, Goldhirsch A, Hayes DF, et al. International web-based consultation on priorities for translational breast cancer research. Breast Cancer Res 2007;9:R81.
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013;24:2206–23.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10.
- Balslev I, Axelsson CK, Zedeler K, et al. The Nottingham prognostic index applied to 9,149 patients from the studies of the Danish breast cancer cooperative group (DBCG). Breast Cancer Res Treat 1994;32:281–90.
- Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 2010;12:207.
- Schwartz AM, Henson DE, Chen D, et al. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER program. Arch Pathol Lab Med 2014;138:1048–52.
- Ehinger A, Bendahl PO, Elston CW, et al. The role of histological grade in discrimination between Luminal A-like and Luminal B-like subtypes in a series of premenopausal breast cancer patients. Virchows Arch. 2014;465:123.
- Ryden L, Jonsson PE, Chebil G, et al. Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: a randomised, controlled trial with long-term follow-up. Eur J Cancer 2005;41:256–64.
- Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 1996;88:1543–9.
- Chebil G, Bendahl PO, Idvall I, et al. Comparison of immunohistochemical and biochemical assay of steroid receptors in primary breast cancer-clinical associations and reasons for discrepancies. Acta Oncol 2003;42:719–25.
- Falck AK, Bendahl PO, Ingvar C, et al. Analysis of and prognostic information from disseminated tumor cells in bone marrow in primary breast cancer: a prospective observational study. BMC Cancer 2012;12:403.
- Malmstrom P, Bendahl PO, Boiesen P, et al. S-phase fraction and urokinase plasminogen activator are better markers for distant recurrences than Nottingham prognostic index and histologic grade in a prospective study of premenopausal lymph node-negative breast cancer. J Clin Oncol 2001;19:2010–19.
- Klintman M, Bendahl PO, Grabau D, et al. The prognostic value of Ki67 is dependent on estrogen receptor status and histological grade in premenopausal patients with node-negative breast cancer. Mod Pathol 2010;23:251–9.
- Gruvberger-Saal SK, Bendahl PO, Saal LH, et al. Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res 2007;13:1987–94.
- Maisonneuve P, Disalvatore D, Rotmensz N, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res 2014;16:R65.
- Engstrom MJ, Opdahl S, Hagen AI, et al. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat 2013;140:463–73.
- Bastien RR, Rodriguez-Lescure A, Ebbert MT, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics 2012;5:44.
- Braun L, Mietzsch F, Seibold P, et al. Intrinsic breast cancer subtypes defined by estrogen receptor signalling-prognostic relevance of progesterone receptor loss. Mod Pathol 2013;26:1161–71.
- Purdie CA, Quinlan P, Jordan LB, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer 2013;110:565–72.
- Prat A, Cheang MC, Martin M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013;31:203–9.
- Boiesen P, Bendahl PO, South Sweden Breast Cancer Group, et al. Histologic grading in breast cancer–reproducibility between seven pathologic departments. Acta Oncol 2000;39:41–5.
- Dalton LW, Page DL, Dupont WD. Histologic grading of breast carcinoma. A reproducibility study. Cancer 1994;73:2765–70.
- Ellis IO, Coleman D, Wells C, et al. Impact of a national external quality assessment scheme for breast pathology in the UK. J Clin Pathol 2006;59:138–45.
- Harris L, Fritsche H, Mennel R, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287–312.
- Polley MY, Leung SC, McShane LM, et al. An international ki67 reproducibility study. J Natl Cancer Inst 2013;105:1897–906.