Abstract
Aim: To evaluate the long-term prognostic impact of age, local treatment and intrinsic subtypes on the risk of local-regional recurrence (LRR) and breast cancer mortality among low-risk patients.
Material and methods: Cohort study with prospectively collected data, balanced five-year age groups, including 514 Danish lymph node negative breast cancer patients diagnosed between 1989 and 1998, treated with mastectomy (N = 320) or breast-conserving therapy (BCT) (N = 194) and without systemic treatment. Intrinsic subtype approximation was performed by combining information on estrogen-, progesterone-, HER2 receptor and Ki67.
Results: The majority of the tumors had a luminal subtype: 70% Luminal-A (LumA), 16% Luminal-B (LumB), and 10% Luminal-HER2 + (Lum-HER2+). The distribution of intrinsic subtypes between younger (≤45 years) and older (>45 years) patients was similar. Intrinsic subtypes had no prognostic impact on the 20-year LRR risk, regardless of age. A distinct 20-year mortality pattern was observed among the younger patients: 11% of patients with LumB tumor died of breast cancer within the first five years after primary surgery, 23% of patients with Lum-HER2+ tumor died within a 5–10-year period, whereas patients with LumA tumor died with a constant low rate throughout the 20-year period. After 20 years of follow-up, patients with LumA tumor had breast cancer mortality comparable to that of patients with LumB tumor (20%) and lower than Lum-HER2+ tumor (39%). Among the older patients, no distinct mortality pattern was observed, and the 20-year breast cancer mortality was not associated with intrinsic subtypes.
Conclusion: Among low-risk patients, 96% of the tumors were Luminal and the distribution of intrinsic subtypes between younger (≤45 years) and older (>45 years) patients was similar. The observed higher frequency of LRR among younger low-risk BCT patients was not associated intrinsic subtype. The 20-year breast cancer mortality was non-significant for LumA tumors among the older patients, whereas among the younger patients, LumA tumors had a comparable mortality with LumB, but lower than for Lum-HER2 + tumors.
It has become evident that young women with breast cancer have a poorer prognosis than older women; their frequency of recurrence is higher [Citation1,Citation2], and their survival lower [Citation1,Citation3–6]. In a recent Danish study of low-risk breast cancer patients enrolled in the Danish Breast Cancer Group (DBCG) 89a-protocol [Citation7], we found that local recurrence was associated with distant metastasis (DM) among younger patients (≤45 years). Moreover, younger patients who received breast-conserving therapy (BCT) had a higher mortality than younger patients who had mastectomy in the DBCG89a cohort treated according to the guidelines at the time. Despite the fact that this study population received no adjuvant treatment, two thirds of the patients were alive and had no recurrence after 20 years, regardless of age.
In the past 15 years, there have been a growing focus on breast tumor heterogeneity, and genomic studies have defined four major intrinsic subtypes of importance: Luminal A (LumA), Luminal B (LumB), HER2-like (HER2+), and basal-like (Basal) [Citation8]. These subtypes have primarily been approximated by combining estrogen receptor status (ER), progesterone receptor status (PR), and HER2 receptor expressions [Citation9,Citation10] determined immunohistochemically. In some cases, ki67 [Citation11–13], tumor grade [Citation14,Citation15], and CK5/6 and EGFR [Citation12,Citation13,Citation16,Citation17] have also been included.
Local failure has been associated with intrinsic subtype approximation in a meta-analysis by Lowery et al. [Citation18]; patients with LumA tumors were found to have a lower risk of local recurrence than patients with Basal/HER2 + tumors. The studies included in the meta-analysis included mainly postmenopausal women and most studies included lymph node positive patients. However, whether intrinsic subtype can be used as prognostic marker for local failure among young and old lymph node negative patients remains unknown.
The 10-year survival has been estimated to fall in the 89%–92% range among lymph node negative patients who have a LumA tumor [Citation10,Citation19], and their survival has been found to be significantly higher than that of patients with other intrinsic subtypes. These studies included both pre- and postmenopausal patients who received different kinds of local treatment (BCT or mastectomy ± radiation therapy). However, in a pooled analysis of very young (<40 years) breast cancer patients [Citation14] from four EORTC-randomized trials, of whom the majority (88%) had undergone BCT, it was reported that patients with LumA tumors had a 10-year overall survival (OS) of 94%. It is noteworthy that the three mentioned papers on lymph node negative patients, all demonstrated the same high survival rate among patients with LumA tumors. This indicates that there may not be any survival difference between young patients who undergo BCT and those having mastectomy if the patients are lymph node negative and have a LumA tumor, although studies with long-term follow-up of more than 10 years are needed to draw any inference.
The aim of the present study was to test if the observed higher incidence of local-regional recurrence (LRR) among younger low-risk patients having BCT was associated with intrinsic tumor subtypes, and furthermore, to test if patients with a LumA tumor had a lower long-term breast cancer mortality compared to other intrinsic subtypes.
Material and methods
Study population
The original study cohort (N = 813) was designed with balanced five-year age groups, based on register data from the DBCG and described in detail previously [Citation7]. The original DBCG89a cohort comprised patients considered as having a low risk of recurrence according to the guidelines at the time. The inclusion criteria were: lymph node negative, tumor size <5 cm, no previous cancer and all histological tumor types except invasive ductal carcinoma grade II/III if the patient was premenopausal. However, the cohort encompassed tumors with characteristics that would be considered as having a high risk of recurrence according to current DBCG guidelines. In order to make the study group more comparable to the present day low-risk group, patients with high-risk features (e.g. invasive lobular carcinomas, grade III) were therefore excluded. Thus, the present cohort came to encompass 514 patients sharing the following characteristics: lymph node negative, tumor size <5 cm, no previous cancer, all histological tumor types (except invasive ductal carcinoma grade II/III or invasive lobular carcinoma grade III) and with successful intrinsic subtype classification of the tumor (Supplementary Figure 1, available online at http://www.informahealthcare.com). All patients had given informed consent to be enrolled in the DBCG 89a protocol [Citation20,Citation21]. Data were collected prospectively for the DBCG database. All patients received partial axillary dissection and were advised to have either mastectomy (with no other treatment) or lumpectomy and whole breast radiotherapy (RT) of the residual breast (48 Gy in 24 fractions + boost of 10–16 Gy in 5–8 fractions) [Citation22]. None of the patients received adjuvant systemic treatment.
As previously described [Citation7], patients were followed with clinical examination biannually for five years and then annually for up to 10 years. Complete 20-year follow-up data (including consecutive registration of all breast cancer events for every patient) were obtained from the Danish Civil Registration System, the National Pathology Register and general practitioners (GP), and by reviewing the patients’ medical records.
Procedures
Formalin-fixed, paraffin-embedded (FFPE) breast carcinoma specimens were available from 726/813 (89%) of the cases (Supplementary Figure 1) and a standard histological hematoxylin/eosin (HE) stained section was made. At the time of diagnosis, only ductal carcinomas were assigned a malignancy grade. All lobular carcinomas were given a histological grade, and cases with grade III were excluded, because they were considered as high-risk tumors [Citation23]. Fifty-four tumors were originally diagnosed as medullary carcinomas, but after histopathological review of the HE sections, they were excluded, because all 54 tumors were considered as invasive ductal carcinoma, grade III according to Ridolfi criteria [Citation24]. The content of tumor tissue was evaluated from HE sections, and cases containing less than 5% of invasive tumor cells were excluded.
From the 532 FFPE samples with sufficient tumor tissue, RNA extraction were performed and expression of ESR1, PGR, ERBB2 and MKI67 were quantified with quantitative real time polymerase chain reaction (described in detail in Supplementary Material, available online at http://www.informahealthcare.com [Citation13,Citation25–27]). If the quantification failed, data from medical records and immunohistochemistry (IHC) was used to determinate ER-, PR- and HER2-status.
Intrinsic subtype approximation was classified for each tumor: LumA (ER + and/or PR+, HER2-, Ki67 low), LumB (ER + and/or PR+, HER2-, Ki67 high), Lum-HER2 + (ER + and/or PR+, HER2+), HER2 + (ER-, PR-, HER2+), and Basal (ER-, PR-, HER2-) [Citation11].
Endpoints and statistical analysis
The endpoints studied were LRR, contralateral breast cancer (CC), DM and breast cancer mortality. LRR was defined as failure in the ipsilateral chest wall, breast, overlying skin, axillary or infraclavicular lymph nodes. CC was defined as tumor growth in the contralateral breast. DM was defined as tumor growth in any other region. Breast cancer mortality was defined as death caused by or with disseminated breast cancer.
Cumulative incidence curves for LRR and breast cancer mortality were plotted using a competing risk model, calculating the time to event as the interval between the date of surgery and the event of interest. In the absence of LRR, the observation time was censored at the earliest of the following competing events: CC, DM, other malignant disease or death. Follow-up was continued until the return date of the letter from the GP, the reading date of the electronic medical records or death, as described previously [Citation7]. In the absence of breast cancer death, CC and other causes of death were recorded as competing events. Patients were censored from follow-up at 1 March 2016.
Crude hazard ratios (HR) were computed for all endpoints using Cox proportional hazards regression. If the assumption of proportional hazard could not be accomplished, a risk difference was calculated using the pseudo-value approach. The assumption of proportional hazards was tested with log–minus log plots and by testing zero slopes of scaled Schoenfeld residuals. The χ2-test was used to compare the distribution of baseline characteristics, intrinsic subtypes, and breast cancer failures among groups. Age was dichotomized using 45 years as the cut-point, based on the pattern of failure described previously [Citation7]. The study population was divided into younger patients (≤45 years) and older patients (>45 years). Level of significance was set to 5% and all estimated p-values were two-sided. All statistical tests were performed using Stata version 12.1 (StataCorp, College Station, Texas, USA).
Results
Among the 514 patients, the younger and the older patients had a similar frequency of BCT, small tumors, histological diagnosis, and intrinsic subtypes (). In total, 70% of the tumors were classified as LumA, 16% as LumB, 10% as Lum-HER2+, 1% as HER2+, and 3% as Basal (). This distribution of intrinsic subtypes did not differ significantly between younger and older patients, between BCT and mastectomy or between different tumor size, but Basal tumors were associated with carcinomas of special type, when compared to LumA (p < 0.001) and LumB (p = 0.05) tumors ().
Table 1. Clinical and pathological characteristics of the study population, comparison of four groups: younger/older and BCT/mastectomy. Younger: ≤45 years; older: >45 years.
Table 2. Clinical and pathological characteristics stratified by intrinsic subtypes.
Within each age group (), patients receiving BCT had a significantly lower frequency of large tumors than patients receiving mastectomy (younger: 9% vs. 24%, p < 0.002, older: 7% vs. 29%, p < 0.001). Among the younger patients, the frequency of different histological diagnosis was similar among those who received BCT compared to those who received mastectomy. In contrast, among the older patients, those who received BCT had a significantly lower frequency of invasive lobular carcinoma (11% vs. 23%, p < 0.03).
Patterns of failure
The proportion of different breast cancer events in relation to intrinsic subtype and histological diagnosis is shown in and , respectively. Overall, younger patients had a significantly higher proportion of LRR (22% vs. 6%, <0.001) as compared to older patients, and the risk of developing DM after or simultaneously with LRR was higher (9% vs. 1%, p < 0.001). Forty percent of the younger patients with LRR developed DM simultaneously or later (59 LRR/24 DM), whereas only 14% of the older patients did (14 LRR/2 DM). Within each age group, the intrinsic subtype () was not associated with a specific failure pattern; LumA tumors had a similar frequency of LRR compared with the other subtypes (both LRR and LRR + DM) and DM. Only four patients had HER2 + tumors; three had no breast cancer events and one developed LRR. Histological diagnosis was not associated with a specific failure pattern among the younger patients (), whereas only those with ductal carcinoma developed LRR among the older patients.
Table 3. Failure pattern as a function of intrinsic subtype and age. Younger: ≤45 years; older: >45 years.
Table 4. Failure pattern as a function of histological diagnosis and age. Younger: ≤45 years; older: >45 years.
Local-regional recurrence
The median follow-up was 14.8 years (range 0.3–24.1). Overall, after 20 years of follow-up, the cumulative incidence of LRR was 15% (95% CI 11–18) (). Younger patients had a higher risk of developing LRR after 20 years than older patients did [risk difference (RD) = 17% (11–23%)]; and among the younger patients, the 20-year risk of LRR was higher after BCT than after mastectomy, RD =19% (7.4–30%) (). BCT patients developed LRR throughout the 20-year period regardless of age. In contrast, the younger mastectomy patients developed LRR within the first 13 years after their breast cancer diagnosis.
Figure 1. Twenty-year loco-regional recurrence as a function of age (A), local treatment (B,C), intrinsic subtypes (D–F) and histological diagnosis (G–I). All patients (A,D,G). Younger (≤45) patients (B,E,H). Older (>45) patients (C,F,I). In the sub-analysis of intrinsic subtype (E,F) HER2 + and Basal tumors have been omitted due to low numbers. Data shown in .
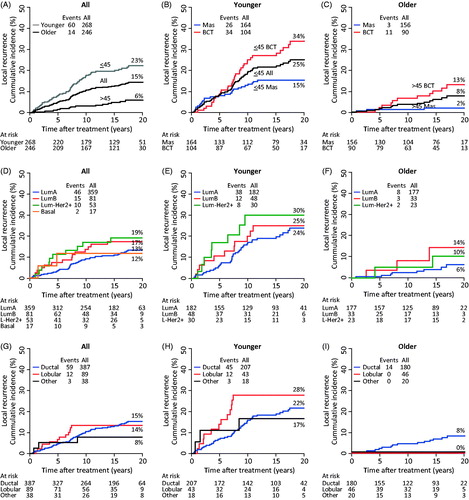
Overall, neither the 10-year nor the 20-year risk of LRR was associated with intrinsic subtypes () and no association was found among the younger patients (, ) or among the older patients (, ).
LRR was not associated with histological diagnosis (), but the recurrence pattern was different. Among the younger patients (), a significantly higher eight-year LRR risk was seen for invasive lobular carcinomas than for invasive ductal carcinomas, RD =17% (2.7–31%), but no such association was found after 20 years. Among the older patients () only those with ductal carcinomas developed LRR.
Breast cancer mortality
The median follow-up on vital status was 19.0 years (range 0.4–26 years). Overall, the 20-year breast cancer mortality was 17% (12–22%), and it was similar in younger and older patients () as well as in BCT patients and mastectomy patients ().
Figure 2. Twenty-year breast cancer mortality as a function of age (A), local treatment (B,C), intrinsic subtypes (D–F) and histological diagnosis (G–I). All patients (A,D,G). Younger (≤45) patients (B,E,H). Older (>45) patients (C,F,I). In the sub-analysis of intrinsic subtype (E,F) HER2 + and Basal tumors have been omitted due to low numbers. Data shown in .
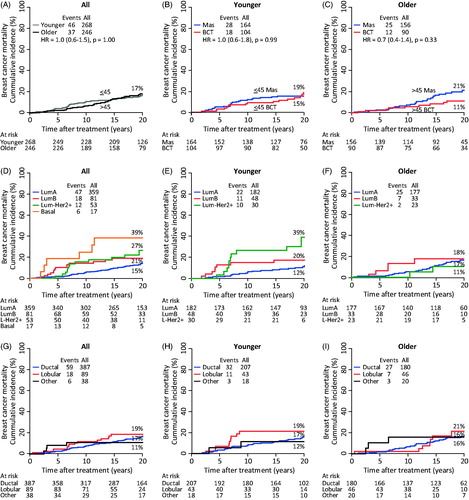
The intrinsic subtypes were associated with a distinct mortality pattern among the younger patients (): 11% of patients with a LumB tumor died of breast cancer within the first five years after primary surgery, 23% of patients with a Lum-HER2 + tumor died within the 5–10 period, whereas patients with a LumA tumor died at a constant low rate throughout the 20-year period, recording a 5-, 10- and 20-year breast cancer mortality of 1.1%, 5.7%, and 12%, respectively. Thus, after 20 year of follow-up, patients with LumA tumor had a breast cancer mortality that was comparable to that of patients with LumB (20%, p = .24) and lower than Lum-HER2 + tumors (39%, p = .02). Among the older patients, no distinct mortality pattern was observed, and the 20-year breast cancer mortality was not associated with intrinsic subtypes (, ).
Histological diagnosis was also associated with a distinct mortality pattern (). A total of 11% (N = 6) of the patients who had carcinomas of special type died within the first 10 years after primary surgery. Among patients with invasive lobular carcinomas the breast cancer mortality pattern was associated with the patients’ age: 19% of the younger patients died within the first 10 years after surgery (), whereas 21% of the older patients died within 10–20 years after surgery (). Patients with invasive ductal carcinomas died at a low and constant rate throughout the 20-year period, recording a 5-, 10- and 20-year breast cancer mortality of 2.7%, 7.4%, and 17%, respectively. Thus after 20 years, the breast cancer mortality was not different between the following histological diagnoses: invasive ductal (17%), invasive lobular (19%), and carcinomas of special type (11%).
Based on the findings described in the paper by Van der Hage et al. [Citation14], a sub-analysis among the younger patients (N = 149) with the best prognosis (tumor ≤2 cm, LumA subtype) was performed. After 22 years the breast cancer mortality was 8% after mastectomy (N = 82), and no patients died of breast cancer within the 15–22-year period after surgery. In contrast the 22-year breast cancer mortality was 17% after BCT (N = 67), eight BCT patients died within the 15–22-year period, and 6/8 had developed LRR more than eight years after primary surgery.
Discussion
Among low-risk patients 96% of the tumors were Luminal and the distribution of intrinsic subtypes between younger (≤45 years) and older (>45 years) patients was similar. Age or intrinsic subtypes had no prognostic impact on the 20-year LRR risk. Among the older patients, the 20-year breast cancer mortality was not associated to intrinsic subtype. In contrast, among the younger patients a distinct mortality pattern associated to intrinsic subtype was observed. After 20 years, patients with LumA and LumB tumors had comparable breast cancer mortality, whereas patients with Lum-HER2 + tumors had a higher mortality. Among patients who had BCT, younger patients with small LumA tumors died of breast cancer more than 15 years after surgery and in the majority of these cases (75%), the patient had developed LRR more than eight years after primary surgery.
The original DBCG89a cohort comprised patients considered as having a low risk of recurrence according to the guidelines at the time. However, the cohort encompassed tumors with characteristics that would be considered as having a high risk of recurrence according to current DBCG guidelines. In order to make the study group more comparable to the present day low-risk group, patients with high-risk features were therefore excluded [Citation7].
The very low frequency of HER2 + and Basal tumors observed in the present study may be caused by the fact that the cohort only included patients with an a priori low risk of recurrences. In our study, only invasive ductal carcinoma of grade I and invasive lobular carcinoma grade I/II was included, whereas all grades were included in other reports of lymph node negative [Citation28,Citation29].
The association between intrinsic subtype and five-year risk of local failure has been investigated in several cohorts in which low- and high-risk patients as well as local treatments and systemic treatments have been mixed. Some studies observed that HER2 + and/or Basal subtype tumors have a significantly higher risk of local failure than LumA tumors [Citation12,Citation15,Citation16,Citation28,Citation30], whereas others found no such association [Citation19,Citation29,Citation31–34]. Only few studies have more than five years of observation time [Citation9,Citation12,Citation19]. In one of them [Citation12], the 10-year risk of local recurrence after BCT was associated with HER2 + tumors. In contrast, no association was found between local recurrence and intrinsic subtypes in a randomized trial of adjuvant systemic treatment, which included 1951 lymph node negative patients [Citation19]; but patients with LumA tumors had a 89% OS after 10 years, which was significantly better than the OS found for the remaining subtypes. A similarly improved 10-year breast cancer-specific survival of 92% among patients with LumA tumors was observed in a study of 887 lymph node negative patients who received no adjuvant systemic treatment [Citation10]. The beforementioned papers [Citation10,Citation19] included both pre- and postmenopausal patients who received different kinds of local treatment (BCT or mastectomy ± RT). However, the same high survival rate (94%) was observed in a pooled analysis of very young (<40 years) breast cancer patients from four EORTC-randomized trials in which the majority (88%) had undergone BCT [Citation14], indicating that young BCT patients with a LumA tumor also have a good prognosis after 10 years.
The overall 10-year breast cancer-specific survival was 94% for patients with LumA tumors. However, after 20 years, the advantages of having of a LumA tumor compared with the other subtypes were non-significant among the older patients (18%). In comparison, the younger patients with LumA tumor (12%) had a significantly lower risk than Lum-HER2 + (39%), but comparable to LumB (20%). A pooled analysis [Citation35], including a mixture of TNM stages observed a similar time-dependent survival pattern; all subtypes had a poorer survival outcome than LumA during the initial years of diagnosis (0–6 years), but women with the LumA subtype had a constant mortality rate throughout the 15-year observation period, leading to a poorer survival for those with LumA tumors than for those with other subtypes after six years. To our knowledge, no other studies have demonstrated 20-year follow-up data for intrinsic subtypes among low-risk breast cancer patients.
The present study was a population-based cohort study, not a randomized clinical trial, and a concern could be that the quality of the data was therefore inferior, as demonstrated when SEER observational data were compared with randomized data [Citation36]. However, follow-up information from patients included in the DBCG 89a-protocol was collected prospectively by the DBCG. Moreover, a study evaluating the implementation of BCT as a routine procedure in Denmark (the DBCG 89-program) reported an equal failure pattern and improved survival in comparison with women from the randomized DBCG 82TM-protocol, evaluating BCT versus mastectomy [Citation37].
As 15% of the patients were excluded due to insufficient amount of tumor material, a potential selection bias could have been introduced if a higher proportion of small tumors were excluded (Supplementary Figure 1), but the clinico-pathological parameters were evenly distributed between patients with and without classification. Also, the approximation of intrinsic subtypes based on the ER-, PR-, and HER2 status has inherent limitations as this method has a reduced set of biomarkers compared with genomic studies, and our classification of intrinsic subtypes does not necessarily apply to the genotype-based subtypes [Citation13,Citation38]. In the present study, luminal tumors (ER + or PR + and HER2-) have been discriminated by the proliferation marker ki-67: LumA (low ki-67, <14%) and LumB (high ki-67) described by Cheang et al. [Citation11]. The usefulness of ki67 is a matter of discussion; the reproducibility is low [Citation39] and the 14% cutoff point have been challenged [Citation40,Citation41]. Other studies have argued that histological grade can be used as well: LumA (grade I/II) and LumB (grade III) [Citation14,Citation15]. In the present study, grade III tumors were excluded to match current DBCG guidelines, so our study would have classified all ER/PR + and HER2- tumors as LumA, if grade was used to discriminate between LumA and LumB tumors.
According to the current 2016 treatment guidelines from DBCG (www.dbcg.dk), almost all of the patients from our study would have received adjuvant anti-hormonal therapy, chemotherapy and/or trastuzumab, which affects the generalizability of our findings to current clinical practice. Due to the increased use of systemic treatment, local failure rates have decreased over the past decades; hence, 10-year local failure risks at 3–5% have been published [Citation42,Citation43]. Therefore, the observed breast cancer mortality difference between younger patients receiving BCT and those receiving mastectomy would probably be lower today. Nevertheless, it is noteworthy that among younger patients with no adjuvant systemic treatment and a low-risk tumor, only BCT patients died of breast cancer in the period from 15 to 22 years after primary surgery, and most of these cases had developed LRR more than eight years after primary surgery. Mastectomy may have a preventive effect among younger patients, protecting them from development of an aggressive breast tumor in the residual breast tissue.
Access to data concerning the long-term effect of BCT and mastectomy in young patients is very limited. Six randomized controlled trials comparing mastectomy and BCT all included a low proportion of young breast cancer patients (12–23%) [Citation44], making it hard to draw any conclusion for this subgroup. Few cohort studies restricted to young breast cancer patients have been published [Citation2,Citation45–49], and they are limited by the inclusion of a mixture of different TNM stages, by the variety of adjuvant systemic treatments offered, and by a median follow-up time of less than 10 years. In contrast, the present study is based on an unbiased cohort (comprising almost all lymph node negative patients below 41 years treated in Denmark in the 1989–1998 period) with complete registration of incident breast cancer and complete follow-up data on LRR and mortality within 20 years. Thus, these data provide a robust estimate of the long-term LRR risk and breast cancer-specific survival in relation to intrinsic subtype, age and local treatment.
Conclusion
Among low-risk patients, 96% of the tumors were Luminal and the distribution of intrinsic subtypes between younger (≤45 years) and older (>45 years) patients was similar. The observed higher frequency of LRR among younger low-risk BCT patients was neither associated to histological diagnosis, nor to intrinsic subtype. Furthermore, the 20-year breast cancer mortality was non-significant for LumA tumors compared to other subtypes among the older patients, whereas among the younger patients, LumA tumors had a comparable mortality with LumB, but lower than for Lum-HER2 + tumors.
Supplementary_Material.pdf
Download PDF (215.1 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Jones HA, Antonini N, Hart AA, et al. Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol. 2009;27:4939–4947.
- Kroman N, Holtveg H, Wohlfahrt J, et al. Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer. 2004;100:688–693.
- Dubsky PC, Gnant MF, Taucher S, et al. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3:65–72.
- Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347.
- El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194.
- de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043.
- Laurberg T, Lyngholm CD, Christiansen P, et al. Long-term age-dependent failure pattern after breast-conserving therapy vs. mastectomy among Danish lymph-node-negative breast cancer patients. Radiother Oncol. 2016;120:98–106.
- Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874.
- Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426.
- Mulligan AM, Pinnaduwage D, Bull SB, et al. Prognostic effect of basal-like breast cancers is time dependent: evidence from tissue microarray studies on a lymph node-negative cohort. Clin Cancer Res. 2008;14:4168–4174.
- Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750.
- Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691.
- Tramm T, Kyndi M, Myhre S, et al. Relationship between the prognostic and predictive value of the intrinsic subtypes and a validated gene profile predictive of loco-regional control and benefit from post-mastectomy radiotherapy in patients with high-risk breast cancer. Acta Oncol. 2014;53:1337–1346.
- van der Hage JA, Mieog JS, van de Velde CJ, et al. Impact of established prognostic factors and molecular subtype in very young breast cancer patients: pooled analysis of four EORTC randomized controlled trials. Breast Cancer Res. 2011;13:R68.
- Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–3891.
- Millar EK, Graham PH, O'Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708.
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374.
- Lowery AJ, Kell MR, Glynn RW, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133:831–841.
- Metzger-Filho O, Sun Z, Viale G, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31:3083–3090.
- Ewertz M, Kempel MM, During M, et al. Breast conserving treatment in Denmark, 1989-1998. A nationwide population-based study of the Danish Breast Cancer Co-operative Group. Acta Oncol. 2008;47:682–690.
- Christiansen P, Al-Suliman N, Bjerre K, et al. Recurrence pattern and prognosis in low-risk breast cancer patients-data from the DBCG 89-A programme. Acta Oncol. 2008;47:691–703.
- Overgaard M, Christensen JJ. Postoperative radiotherapy in DBCG during 30 years. Techniques, indications and clinical radiobiological experience. Acta Oncol. 2008;47:639–653.
- Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27:49–61.
- Ridolfi RL, Rosen PP, Port A, et al. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365–1385.
- Tramm T, Hennig G, Kyndi M, et al. Reliable PCR quantitation of estrogen, progesterone and ERBB2 receptor mRNA from formalin-fixed, paraffin-embedded tissue is independent of prior macro-dissection. Virchows Arch. 2013;463:775–786.
- Kyndi M, Sørensen FB, Knudsen H, et al. Tissue microarrays compared with whole sections and biochemical analyzes. A subgroup analysis of DBCG 82 b&c. Acta Oncol. 2008;47:591–599.
- Andersen J, Bentzen SM, Poulsen HS. Relationship between radioligand binding assay, immunoenzyme assay and immunohistochemical assay for estrogen receptors in human breast cancer and association with tumor differentiation. Eur J Cancer Clin Oncol. 1988;24:377–384.
- Albert JM, Gonzalez-Angulo AM, Guray M, et al. Estrogen/progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a,bN0 breast cancer. Int J Radiat Oncol Biol Phys. 2010;77:1296–1302.
- Selz J, Stevens D, Jouanneau L, et al. Prognostic value of molecular subtypes, ki67 expression and impact of postmastectomy radiation therapy in breast cancer patients with negative lymph nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2012;84:1123–1132.
- Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat. 2011;127:713–720.
- Truong PT, Sadek BT, Lesperance MF, et al. Is biological subtype prognostic of locoregional recurrence risk in women with pT1-2N0 breast cancer treated with mastectomy? Int J Radiat Oncol Biol Phys. 2014;88:57–64.
- Sanpaolo P, Barbieri V, Genovesi D. Prognostic value of breast cancer subtypes on breast cancer specific survival, distant metastases and local relapse rates in conservatively managed early stage breast cancer: a retrospective clinical study. Eur J Surg Oncol. 2011;37:876–882.
- Mersin H, Gulben K, Berberoglu U, et al. Prognostic factors affecting postmastectomy locoregional recurrence in patients with early breast cancer: are intrinsic subtypes effective?. World J Surg. 2011;35:2196–2202.
- Demirci S, Broadwater G, Marks LB, et al. Breast conservation therapy: the influence of molecular subtype and margins. Int J Radiat Oncol Biol Phys. 2012;83:814–820.
- Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279.
- Henson KE, Jagsi R, Cutter D, et al. Inferring the effects of cancer treatment: divergent results from early breast cancer trialists' collaborative group meta-analyzes of randomized trials and observational data from SEER registries. J Clin Oncol. 2016;34:803–809.
- Lyngholm CD, Laurberg T, Alsner J, et al. Failure pattern and survival after breast conserving therapy. Long-term results of the Danish Breast Cancer Group (DBCG) 89 TM cohort. Acta Oncol. 2016;55:983–992.
- Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–5232.
- Polley MY, Leung SC, McShane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105:1897–1906.
- Denkert C, Loib S, Müller BM, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 2013;24:2786–2793.
- Bustreo S, Osella-Abate S, Cassoni P, et al. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157:363–371.
- Mannino M, Yarnold JR. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld? Radiother Oncol. 2009;90:14–22.
- Bartelink H, Bourgier C, Elkhuizen P. Has partial breast irradiation by IORT or brachytherapy been prematurely introduced into the clinic?. Radiother Oncol. 2012;104:139–142.
- Cao JQ, Olson RA, Tyldesley SK. Comparison of recurrence and survival rates after breast-conserving therapy and mastectomy in young women with breast cancer. Curr Oncol. 2013;20:e593–601.
- Bantema-Joppe EJ, de ML, Visser O, et al. Early-stage young breast cancer patients: impact of local treatment on survival. Int J Radiat Oncol Biol Phys. 2011;81:e553–e559.
- van der Sangen MJ, van de Wiel FM, Poortmans PM, et al. Are breast conservation and mastectomy equally effective in the treatment of young women with early breast cancer? Long-term results of a population-based cohort of 1,451 patients aged ≤40 years. Breast Cancer Res Treat. 2011;127:207–215.
- Cao JQ, Truong PT, Olivotto IA, et al. Should women younger than 40 years of age with invasive breast cancer have a mastectomy? 15-year outcomes in a population-based cohort. Int J Radiat Oncol Biol Phys. 2014;90:509–517.
- Mahmood U, Morris C, Neuner G, et al. Similar survival with breast conservation therapy or mastectomy in the management of young women with early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;83:1387–1393.
- Jeon YW, Choi JE, Park HK, et al. Impact of local surgical treatment on survival in young women with T1 breast cancer: long-term results of a population-based cohort. Breast Cancer Res Treat. 2013;138:475–484.
