Abstract
Background: The risk stratification currently applied prior to curative treatment for localized prostate cancer (PC) does not take into account comorbidity or age. Therefore, we investigated the impact of comorbidity on overall survival (OS) in PC patients treated with external beam radiotherapy (EBRT) and high-dose rate (HDR) brachytherapy boost.
Material and methods: At a single center, 611 consecutive patients diagnosed with localized PC from 1998 to 2004 underwent definitive EBRT (50 Gy) and HDR brachytherapy boosts (2 × 10 Gy) combined with neoadjuvant total androgen blockade. Comorbidity was assessed with the Charlson comorbidity score. The impact of risk factors on OS and disease-free survival (DFS) was calculated using Cox proportional hazard ratios. Risk groups were defined as follows: low-risk PC: PSA <10, WHO grade 1 and T stage 1; high-risk PC: PSA >20 and/or WHO grade 3 and/or T stage 3a; intermediate-risk PC representing patients who did not fit either the low- or high-risk PC group.
Results: Mean age in the study cohort was 66.4 years, and 51% of the patients reported some degree of comorbidity. Divided into risk groups 8.2% were categorized as low-risk, 64% as intermediate-risk and 27.8% as high-risk PC. Overall 10-year survival was 72.2%, and 89% of the patients were relapse-free. In the univariate and multivariate analyses using Cox proportional hazard ratios, age, comorbidity and T stage were statistically significant predictors of OS: hazard ratios 1.56, 1.44 and 1.2 (p-values .002, .04 and .05), respectively. WHO grade, PSA at diagnosis, T stage and comorbidity were also significant predictors of DFS (p-values .0001, .0001, .009 and .003, respectively).
Conclusion: Comorbidity assessed with the Charlson score predicts OS in patients with localized PC treated with curative intent using combined EBRT and HDR brachytherapy boost, and should be considered when making decisions before radical treatment.
Prostate cancer (PC) is the most common malignancy in men in the western world, and 50% of PC patients are over the age of 70 years at diagnosis. Until recently, eligibility for radical treatment of PC was restricted to patients with a life expectancy of ≥10 years. Today, men with aggressive PC are generally considered to benefit from curative treatment up to the age of 80 years, if their life expectancy is ≥5 years [Citation1,Citation2], and this standard has now been translated into changes in geriatric oncology guidelines for high-risk PC [Citation3]. Nevertheless, several studies have suggested that men over the age of 70 years who have aggressive PC but are otherwise healthy are frequently offered suboptimal treatment due to underestimation of their life expectancy [Citation4,Citation5]. In contrast, men with low-risk PC and advanced comorbidity are at risk of overtreatment [Citation6,Citation7]. Comorbidity could be measured prospectively with physical examination and interviews or retrospectively be retrieved from medical records. Various techniques have been adopted to evaluate comorbidity and its impact on survival, and the approach most widely used in this context is the Charlson Comorbidity Index (CCI). This scoring system was first described in 1987, and is a weighted comorbidity index that assesses 19 comorbid conditions [Citation8]. In Denmark, Nguyen-Nielsen et al. [Citation9] used the CCI score to assess the influence of comorbidity on overall survival (OS) in PC patients in a population-based cohort study covering the period 2000–2011 [Citation9], and they concluded that comorbidity was a negative prognostic factor. Those authors found that, in general, OS improved in the PC patients during the study period, although the improvement was modest among men with high comorbidity. Furthermore, comparison of the men without comorbidity and those with heavy comorbidity (CCI score ≥3) revealed a four-fold increase in mortality risk at five years in the latter group. No detailed information regarding PC risk groups or treatment was available in that investigation. For patients treated with prostatectomy, comorbidity is a validated and established predictive factor for survival after treatment [Citation10,Citation11]. Only a few studies have addressed the possibility of comorbidity being a predictive factor in patients who receive radiotherapy with curative intent [Citation12,Citation13].
During recent decades, several new techniques have been developed to safely deliver radiotherapy with dose escalation to increase tumor control and improve outcome [Citation14] including intensity-modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT), combinations with brachytherapy boosts and protons. Gandaglia and coworkers [Citation15] recently used Charlson scoring in a population-based study of a cohort of PC patients treated with IMRT, and the results showed that survival status was improved only in individuals with high-risk disease and no comorbidity. Using the experience from a single institution, we conducted a retrospective investigation to evaluate the impact of comorbidity, age and tumor aggressiveness on OS and disease-free survival (DFS) in PC patients treated with conformal external beam radiotherapy (EBRT) combined with a high-dose rate (HDR) brachytherapy boost.
Material and methods
Patient cohort
The study cohort consisted of all consecutive patients (n = 611) treated with EBRT combined with two fractions of ultrasound-guided transperineal HDR brachytherapy and neo-adjuvant total androgene blockade for localized PC, from May 1998 through August 2004, at Karolinska University Hospital in Stockholm, Sweden. Inclusion and exclusion criteria, are shown in . All patients had a biopsy-confirmed prostatic adenocarcinoma before treatment. During the period 1998–2000, aspiration biopsies were performed for cytological diagnosis, and grading was done using the WHO criteria [Citation16]. Starting at the beginning of 2000, transrectal ultrasound-guided prostate biopsies were gradually introduced, and the grading was done using the Gleason score. To enable comparison of the biopsy results, a transformation was performed according to guidelines stipulated at the local pathology laboratory at the onset of the present study: a Gleason score of ≤5 denoted low-grade cancer (WHO grade 1), and a Gleason score of ≥4 + 3=7 corresponded to high-grade cancer (WHO grade 3). T stage was defined according to the 1992 TNM classification system, and patients with ≥ T3b (palpable growth in the vesicula seminalis) were not considered for combined radiotherapy [Citation17]. Surgical lymph node dissection was performed in patients with at least one of the following risk criteria: PSA >20 ng/ml, high-grade PC (WHO grade 3) or a T3a tumor. In addition, M stage was assessed by bone scan. Only patients with negative lymph node samples and negative bone scans were included in our analysis. The patients were divided into PC risk groups according to the following criteria: low-risk PC designating those with PSA <10, WHO grade 1,and T stage 1; high-risk PC denoting those with PSA >20 and/or WHO grade 3 and/or T stage 3a; intermediate-risk PC representing patients who did not fulfill the criteria for either low- or high-risk PC. The intermediate-risk group included 49 patients with missing T stage.
Table 1. Inclusion and exclusion criteria.
All patients had their controls at Karolinska University Hospital. The follow-up comprised physical examination including digital rectal examination, and blood sample analyses, including PSA every three months the first year, every six months during the next two years, and thereafter annually for up to 10 years after the curative treatment. PSA relapse was defined as a PSA level of nadir +2 ng/ml in order to exclude patients with PSA bounce. All patients with PSA relapse underwent fine-needle aspiration biopsies of the prostate residual mass and magnetic resonance tomography of the pelvis to discriminate between local relapse and disseminated disease. Patients who died were categorized as follows: those with biologically no evidence of disease (bNED) were defined as deaths without PC; those with a PSA relapse without hormonal treatment or with no sign of progression during such treatment were classified as deaths with PC; those with PSA progression during hormonal treatment or PC metastases were defined as deaths caused by PC.
Radiotherapy
The radiotherapy technique applied in the present study has been described previously in detail [Citation18]. Briefly, the target dose of the EBRT was 50 Gy in daily 2 Gy fractions, five days a week, covering the prostate gland and seminal vesicles with a 2-cm margin, except dorsally where the margin was 1.5 cm. The HDR brachytherapy target dose was 20 Gy delivered in two 10 Gy fractions two weeks apart, covering the prostate gland and base of the vesicles with a 3 mm margin.
Comorbidity assessment, Charlson Comorbidity Index
Before their first visit to the clinic, the patients completed an ad hoc constructed questionnaire, with open questions, designed to obtain information concerning previous hospitalization, present comorbidities and ongoing medication. The questionnaire results were discussed and complemented with information obtained by the physicians at the patients’ first visit and noted in the medical chart. The CCI scores were retrospectively calculated from the patients’ questionnaire self-assessments and the medical charts.
As mentioned above, the CCI was first described in 1987. The score was derived from the one-year mortality among 559 medical admissions to a single hospital unit in 1984. This index is well validated and assesses 19 comorbid conditions [Citation8], each of which is assigned a score that is weighted according to the relative risk of death associated with that particular condition. In 1994, the CCI was modified to include adjustment for age [Citation19].
Statistics
The survival analyses were performed using the Kaplan-Meier method, and the log-rank test was applied to demonstrate differences in survival times. DFS time was measured from the start of the first brachytherapy to the final follow-up or the date of death or PSA failure, whichever occurred first. OS time was measured from the start of brachytherapy to the date of death or the last follow-up. The Cox proportional hazard model was used to quantify the relationships between PSA, WHO grade, T stage, comorbidity, death and DFS. To further clarify the significance of each factor the analysis was complemented with a stepwise model. The Kruskal-Wallis test was used to compare means. Analyses of count and frequency data were performed with the χ2-test. All statistical analyses were conducted using Stata 11 software. p-Values ≤.05 were considered statistically significant.
Results
Patient characteristics
In all, 611 patients were included in this study, and in December 2014 the mean follow-up time was 9.6 years (range 0.3–15.5 years). There were 34 patients still alive that had a follow-up shorter than five years and their mean follow-up time was three years (range 1–4.9 years). Patient characteristics according to age, T stage, WHO grade and PSA level at diagnosis are shown in . At the time of treatment, 253 (41.4%) patients were <65 years of age and 358 (58.6%) were ≥65 years. At the time of diagnosis, one third of the patients were >70 years. Categorization of the patients according to PC risk groups, as described above, showed 64% in the intermediate-risk group compared to 8.2% and 27.8% in the low- and high-risk groups, respectively.
Table 2. Patient characteristics.
Comorbidity
Overall, 312 (51%) patients reported some degree of comorbidity. The most frequent comorbidities were hypertension (31%), ischemic heart disease (15.4%) and diabetes mellitus (9.3%). When including the age component, the CCI scores were as follows: 0 (n = 0), 1–2 (n = 423) and ≥3 (n = 188). No difference in average age was observed between patients without reported comorbidity (n = 299) and those with such conditions (65.7 and 66.4 years, respectively). Dichotomization at age 65 years showed that, at the time of treatment, no comorbidity was reported by 53% of those aged <65 years (n = 253) compared to 46% of those aged ≥65 years (n = 358).
Overall survival
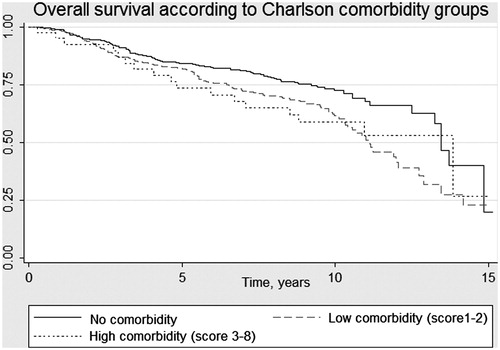
A total of 440 (72.2%) of the 611 patients in the initial cohort were still alive at the time of data collection for the study. Death occurred in 171 patients (27.8%) during follow-up; death was due to PC in 37 (22%) of those men and 17 (10%) died after relapse without hormonal treatment or without progression during hormonal treatment. The majority of patients (n = 117, 68%) died from other causes with no evidence of PC recurrence. Sixty-six patients (22%) died in the group with no comorbidity, compared to 89 (32%) and 16 (38%) in the groups with CCI scores of 1–2 and ≥3, respectively (p = .009). In the group with CCI scores of ≥3, all deaths were due to causes other than PC, whereas PC was the cause of death in 27% of the patients in the group with no comorbidity (p = .013). The mean follow-up time from date of treatment to death for the whole group was 7.2 years, range 0.3–15 years and 5.4 years, range 0.3–13.8 years for the men with; ≥3 comorbidities. OS according to CCI scoring, without the age factor, is illustrated in .
Cox regression univariate analyses yielded statistically significant prognostic information regarding OS in relation to age, comorbidity and T stage, with hazard ratios (HRs) of 1.73, 1.44 and 1.28 (p-values .0006, .003 and .03), respectively. PSA levels at diagnosis (HR 1.21) and WHO grade (HR 1) were not statistically significant predictors of OS (p-values .06 and .97, respectively). PSA at diagnosis analyzed as a continuous variable or dichotomized at 10 yielded the same result. In the multivariate analyses, age, comorbidity and T stage remained independently significant predictors of OS, with HRs of 1.73, 1.46 and 1.24 (p-values .002, .0004 and .05), respectively (). Using a stepwise regression model to evaluate the impact of each prognostic factor further, revealed age and comorbidity as the only significant predictors of OS, HRs of 1.8 and 1.5 (p-values .002 and .004), respectively.
Table 3. Results of univariate and multivariate analysis of overall survival, taking into account age, T stage, WHO grade, PSA level at diagnosis, and comorbidity.
The same results were obtained when statistics were recalculated excluding the 49 patients with missing T stage (data not shown).
Disease-free survival
Among the 440 patients still alive when the database was closed in December 2014, 393 (89%) were relapse-free. In Cox regression univariate analyses, the clinical features PSA level at diagnosis, T stage, WHO grade and comorbidity provided statistically significant prognostic information regarding DFS (p-values .0001, .0001, .009 and .003, respectively), and those findings were confirmed in multivariate analyses (). As expected, age did not affect DFS (p-values .1 and .43). The results were confirmed using a stepwise regression model.
Table 4. Results of univariate and multivariate analysis of DSF, taking into account age, T stage, WHO grade, PSA level at diagnosis and comorbidity.
Discussion
In this retrospective single-center study of patients treated with modern dose-escalated radiotherapy for localized PC, comorbidity assessed using the CCI was found to be a significant independent predictor of OS, as well as age and T stage at 10-year follow-up. PSA level at diagnosis and WHO grade were not predictors of OS in this study. PSA, T stage WHO grade and comorbidity were furthermore statistically significant predictors of DFS.
The influence of comorbidity on outcome in the context of different treatment modalities has been evaluated in several cohort studies, the results of which support comorbidity as an important predictor of OS in men with PC [Citation10,Citation13,Citation14,Citation20]. However, only a few investigations have addressed the impact of comorbidity on OS in PC patients treated with modern dose-escalated radiotherapy. Tendulkar et al. [Citation20] assessed causes of death in a high-risk PC population (n = 660) treated with IMRT to 78 Gy and observed that comorbidity and age were the only predictors of OS. Those findings are supported by our results, although in our study we evaluated PC patients in all risk groups who were treated with combined EBRT and HDR brachytherapy. In addition, it should be noted that T stage was also a statistically significant predictor of OS in our assessment. Several studies have found that there is a tendency for physicians to base their decisions concerning PC treatment solely on chronological age and/or the aggressiveness of the disease [Citation4,Citation21]. In our material, 171 patients died, and 68.8% of the deaths were due to causes other than PC, although the intension was to only treat men with a life expectancy of 10 years or more. All of the patients in the group with ≥3 comorbidities died from causes other than PC.
The OS observed in our study was 72.2% at a mean of 10-years follow-up. However, the range of follow-up time from date of treatment to death was wide, 0.3–15 years for the whole cohort and 0.3–13.8 years for patients with ≥3 comorbidities, indicating that some comorbidities may be more important for predicting mortality. Froehner et al. [Citation22] investigated a group comprising 1910 consecutive patients treated with prostatectomy in 2001–2004 to determine the impact of individual comorbidities on OS in such patients. The results showed that, for the entire cohort, predictive value regarding OS was provided by eight of the 19 conditions included in the CCI: congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes, hemiplegia, moderate or severe renal disease, diabetes with end organ damage, moderate or severe liver disease, and other metastatic solid tumors. However, there were some differences between the age groups in which comorbid conditions were of importance, and Froehner and coworkers suggested that these disparities might have been due to positive selection of individuals in the older age group.
As expected, we found that WHO grade, PSA at diagnosis and T stage were all strong and independent predictive factors of DFS. Surprisingly, comorbidity also predicted DFS. To explain this finding, we compared the various comorbidity groups with respect to differences in fulfillment of treatment and the proportion of patients with high-risk PC, but this did not reveal any statistically significant disparities between the groups. Notwithstanding, that a larger proportion of men in the group with high comorbidity had PSA levels >20 ng/ml at diagnosis, which seems to suggest an increased risk of recurrence in this group. Another plausible explanation is that certain comorbid conditions, such as ischemic heart disease and diabetes, could have caused decreased oxygen tension in tissues, which would have contributed to reduced radiosensitivity because hypoxic tumors are more resistant to radiation treatment [Citation23]. However, in a study evaluating the effect of tumor control in male smokers treated with definitive brachytherapy for PC another factor affecting the oxygenation of tissues was investigated and no difference in DFS were found between smokers and non-smokers [Citation24]. Prospective studies are needed to further elucidate this issue.
Strengths and limitations
The present study has several strengths. First of all, the mean follow-up time was close to 10 years. As 10 years life expectancy is the generally agreed limit for clinical intervention in this patient group, results derived from studies with an actual follow-up time of 10 years, can be regarded as reliable. In addition, the work was conducted at a single high-volume center, which reduced inter-individual differences in staging, treatment procedures and follow-up to a minimum. Furthermore, a substantial number of patients were included compared with other studies addressing the same issue.
The main limitations are the retrospective design and the risk of bias in selection of men to the patient cohort. Several authors report that patients can be assigned to different treatment modalities based on age and comorbidity, for example older patients, patients with comorbidity and patients with locally advanced disease are more often treated with radiotherapy or watchful waiting [Citation25]. In our cohort of patients treated with EBRT combined with a HDR brachytherapy boost, we found no statistically significant differences in comorbidity between the age groups. This could be interpreted that the comorbidity score has affected the treatment decision and the selection of patients with comorbidity to radiotherapy instead of surgery, supporting the results of previous studies. However, high-risk PC was slightly more common in older patients, whereas few men had a CCI score of ≥3 (n = 40), observations that probably reflect a selection of healthier men for curative treatment, in the older age group.
Conclusion
To our knowledge, this is the first study to describe the impact of comorbidity in a large group of PC patients homogeneously treated with dose-escalated radiotherapy including a HDR brachytherapy boost. We conclude that comorbidity assessed with the Charlson score represents a valuable tool for predicting long-term survival in PC patients treated with combined radiotherapy. To avoid over and under treatment of men with PC, comorbidity should be considered in the decision making process regarding curative treatment.
Disclosure statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Schwartz KL, Alibhai SM, Tomlinson G, et al. Continued under treatment of older men with localized prostate cancer. Urology. 2003;62:860–865.
- Hall WH, Jani AB, Ryu JK, et al. The impact of age and comorbidity on survival outcomes and treatment patterns in prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:22–30.
- Droz JP, Aapro M, Balducci L, et al. Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol. 2014;15:e404–e414.
- Daskivich TJ, Chamie K, Kwan L, et al. Matching tumor risk with aggressiveness of treatment in men with multiple comorbidities and early-stage prostate cancer. Cancer. 2013;119:3446–3453.
- Abdollah F, Schmitges J, Sun M, et al. Comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol. 2012;19:836–844.
- Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058–2066.
- Jha GG, Anand V, Soubra A, et al. Challenges of managing elderly men with prostate cancer. Nat Rev Clin Oncol. 2014;11:354–364.
- Charlson ME, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Nguyen-Nielsen M, Nørgaard M, Jacobsen JB, Borre M, Thomsen RW, Søgaard M. Comorbidity and survival of Danish prostate cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol. 2013;5(Suppl 1):47–55.
- Froehner M, Koch R, Litz RJ, et al. Interaction between age and comorbidity as predictors of mortality after radical prostatectomy. J Urol. 2008;179:1823–1829.
- Alibhai SM, Leach M, Tomlinson G, et al. 30-day mortality and major complications after radical prostatectomy: influence of age and comorbidity. J Natl Cancer Inst. 2005;97:1525–1532. Erratum in J Natl Cancer Inst. 2007;99:1648.
- Post PN, et al. The independent prognostic value of comorbidity among men aged o75 years with localized prostate cancer: a population-based study. BJU Int. 2001;63:821–826.
- Abdollah F, Sun M, Schmitges J, et al. Competing-risks mortality after radiotherapy vs. observation for localized prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2012;84:95–103.
- Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405–1418.
- Gandaglia G, Karakiewicz PI, Briganti A, et al. Intensity-modulated radiation therapy leads to survival benefit only in patients with high-risk prostate cancer: a population-based study. Ann Oncol. 2014;25:979–986.
- Mostofi FK, Sesterhenn I, Sobin LH, editors. Histological typing of prostate tumours. Geneva: World Health Organization 1982:15–23.
- UICC 1992 Classification.
- Kälkner KM, Wahlgren T, Ryberg M, et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 2007;46:909–917.
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251.
- Tendulkar RD, Hunter GK, Reddy CA, et al. Causes of mortality after dose-escalated radiation therapy and androgen deprivation for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2013;87:94–99.
- de Camargo Cancela M, Comber H, Sharp L. Age remains the major predictor of curative treatment non-receipt for localized prostate cancer: a population-based study. Br J Cancer. 2013;109:272–279.
- Froehner M, Koch R, Litz RJ, Oehlschlaeger S, Twelker L, Hakenberg OW, Wirth MP. Detailed analysis of Charlson comormidity score as predictor of mortality after radical prostataectomy. Urology. 2008;72:1252–1257.
- Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004;43:396–403.
- Taira AV, Merrick GS, Galbreath RW, et al. Prognostic importance of tobacco use in men receiving definitive prostate brachytherapy. Brachytherapy. 2012;11:446–451.
- Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158:709–717.