Abstract
Background: Docetaxel in combination with cisplatin and 5-fluorouracil (5-FU) is one of several standard chemotherapy regimens for patients with advanced gastro-esophageal adenocarcinoma (aGEA) in Europe. To enable outpatient treatment, we evaluated the maximum tolerated dose (MTD), recommended dose (RD), dose limiting toxicity (DLT) and safety of docetaxel in combination with oxaliplatin (O) and S-1 (DOS) in Caucasian patients with aGEA.
Methods: We present final results of two parallel phase 1/2a studies (3 + 3 design). Escalating doses of docetaxel and S-1 with fixed dose O were given for 18 weeks every second week (DOS2w) or every third week (DOS3w) followed by S-1 maintenance therapy.
Results: Thirty-four patients (18 in DOS2w and 16 in DOS3w) were enrolled between October 2013 and June 2015. Median age was 65 years (range 49–78). DLT was most often febrile neutropenia. Most common severe non-hematological adverse events were diarrhea (9%) and fatigue (6%). The RD of DOS3w was: docetaxel 50 mg/m2, O 100 mg/m2 and S-1 25 mg/m2 twice daily and of DOS2w was: docetaxel 40 mg/m2, O 70 mg/m2 and S-1 35 mg/m2 twice daily. Overall, response rate was 56%; median progression-free survival was 9.1 months; and median overall survival was 13.2 months in 34 patients.
Conclusions: At the RD, DOS2w and DOS3w showed an acceptable safety profile in patients with aGEA.
Clinical trials ID: NCT-01928524 and EudraCT 2012-005187-10
Palliative treatment prolongs survival and improves quality of life in patients with advanced gastro-esophageal adenocarcinoma (aGEA), but there is no worldwide consensus on the optimal combination regimen [Citation1,Citation2]. Fluoropyrimidine- and platinum-based therapies are the backbones of treatment for aGEA and substitution of cisplatin (C) with oxaliplatin (O) and infusion of 5-fluorouracil (5-FU) with an oral capecitabine (X) ease the administration burden for patients with at least comparable efficacy [Citation3]. Triple regimens with docetaxel have shown to be more effective than C/5-FU (CF) regimens [Citation4], however, the triple regimen with docetaxel and CF (DCF) was also more toxic, as would be expected.
S-1 (Teysuno®) is an oral, combination product comprising tegafur (FT), a prodrug of 5-FU, and two modulators of 5-FU metabolism, gimeracil (CDHP) and oteracil (Oxo), in a 1:0.4:1 molar ratio (FT:CDHP:Oxo). S-1 administered in combination with C has been approved in Europe as an alternative regimen to CF for the treatment of aGEA after having demonstrated non-inferiority in efficacy to CF, but with a significantly better safety profile [Citation5,Citation6]. Combinations of O [Citation7] or docetaxel with S-1 [Citation8] have become widely used doublets in Japan. In a few Asian phase I and II studies, recommended doses (RD) for DOS every three weeks (D 50–52 mg/m2 Day 1, O 100–105 mg/m2 Day 1, S-1 80 mg/m2 Days 1–14) have been established [Citation9–11], but DOS with establishment of RD has not been investigated in Caucasian patients. We have previously established a RD of DOX every three weeks in a phase I dose-finding trial in 23 patients with aGEA: docetaxel (50 mg/m2), short-time infusion of O (100 mg/m2) and continuous X (625 mg/m2 twice daily) [Citation12,Citation13]. Based on the Asian data [Citation9–11] and our recent phase I study [Citation12,Citation13], we wanted to establish a RD for an ‘every three weeks’ DOS regimen (DOS3w). However, promising data from the GATE study [Citation14] inspired us to also evaluate an ‘every two weeks’ regimen (DOS2w). Therefore, we designed this dose-finding study and here we present the findings from two parallel phase 1/2a studies exploring DOS2w or DOS3w in Caucasian patients with aGEA.
Patients and methods
Study design and treatment
Two parallel phase 1/2a studies with 3 + 3 design with either DOS given every second week or every third week in Caucasian patients (). Patients were included into DOS2w and DOS3w in sequence. Doses of chemotherapy were selected based on experience from our recent phase I study [Citation12,Citation13]. Patients received escalating doses of docetaxel as a 60-minute infusion on Day 1 with O as a 30-minute infusion on Day 1. S-1 was administered orally twice daily, with the first dose administered in the evening of Day 1 of each cycle. Capsules were taken with water at least 1 hour before or 1 hour after a meal.
Table 1. Dose-escalation scheme with DLT and number of patients at each dose level.
In the DOS2w group: patients received escalating doses of docetaxel (30–50 mg/m2 on Day 1), fixed doses of O (70 mg/m2 on Day 1) and S-1 (30–35 mg/m2/day twice daily orally on Days 1–7) every two weeks.
In the DOS3w group: patients received escalating doses of docetaxel (40–60 mg/m2 on Day 1), fixed doses of O (100 mg/m2 on Day 1) and S-1 (25 mg/m2/day twice daily orally on Days 1–14) every three weeks.
DOS was planned to be given for 18 weeks (six cycles of DOS3w or nine cycles of DOS2w) followed by maintenance treatment with S-1 monotherapy (30 mg/m2/day twice daily on Days 1–14 every three weeks) until progression, unacceptable toxicity, patient refusal or physician recommendation. Sequential cohorts of patients were included at progressively higher dose levels.
Patient selection
Patients were required to have histologically confirmed adenocarcinoma of the lower esophagus, gastro-esophageal junction or stomach not amenable to surgical resection (non-resectable or metastatic). Eligibility criteria included measurable or non-measurable disease; WHO performance status 0–1; age ≥18 years; no prior chemotherapy other than adjuvant/perioperative chemotherapy completed at least six months before inclusion; adequate bone marrow function (neutrophil count ≥1.5 × 109/l; platelets ≥100 × 109/l); adequate hepatic function (serum bilirubin ≤1.5 × upper normal limit (UNL); transaminase ≤3 × UNL (in cases of liver metastases there was no upper limit for transaminases); adequate renal function (calculated creatinine clearance ≥60 ml/min by the Cockcroft and Gault formula). Other inclusion criteria were: treatment start within eight days after inclusion; ability to tolerate oral medication; no peripheral neuropathy; no co-existent severe medical illness; no sign of brain metastases; no concomitant treatment with other anticancer drugs. Females were not included if they were pregnant or lactating.
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the ICH Harmonized Tripartite Guidelines for Good Clinical Practice (GCP), and applicable local and national laws and regulations. The study was approved by the local ethics committee and the Danish Health Authority (EUDRACT 2012-005187-10). Signed informed consent was obtained from all participants before study entry.
Evaluation of toxicity, dose limiting toxicity and dose adjustment
Toxicity was measured and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 3.0). Subjective symptoms, physical examination, WHO performance status, hematology (also Day 10 of first course) and adverse events were recorded before starting each treatment cycle. Dose limiting toxicity (DLT) was evaluated after the first cycle and defined as the occurrence of any of the following toxicities: Grade ≥3 non-hematological toxicity; febrile neutropenia; grade 4 neutropenia >7 days (absolute neutrophil count <0.5 × 109/l); grade 4 thrombocytopenia (platelet count <25 × 109/l); grade ≥3 infection or delay of treatment for more than two weeks due to side effects. If a patient experienced a DLT at a certain dose level the dosage of all drugs for that patient was reduced to 75%.
In case of DLT among one of three patients during the first cycle additional three patients were added at the respective dose level. Dose escalation was continued if none of three or one of six patients experienced DLT. The maximum tolerated dose (MTD) was defined as the dose level that produced DLT in two or more patients in either the initial cohort of three or the expanded cohort of six. The RD was defined as one dose level below the MTD.
Patient evaluation
Assessment of medical history, physical examination, evaluation of WHO performance status, complete blood count and biochemical tests were performed before treatment start and prior to each cycle. A computed tomography (CT) scan of thorax and abdomen was carried out at baseline and after every three (DOS3w) or four (DOS2w) cycles. Response was assessed by investigators according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). After completion of treatment patients were followed every third month until progression or death.
Statistical methods
The primary endpoint of this phase 1/2a study was to determine the RD of the DOS2w and DOS3w dosing regimens. In addition, overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) were evaluated. Non-parametric statistics were applied. PFS and OS with 95% confidence interval (CI) were calculated according to the Kaplan-Meier method and updated in October 2015. Data were recorded and analyzed in a Medlog® database.
Results
All patients were included and treated at a single institution, Odense University Hospital, Denmark. Of the 35 patients screened, only one was deemed to be ineligible, and 34 were enrolled and treated (). The first and last patients were enrolled in October 2013 and June 2015, respectively. The median age of patients was 65 years (range 49–78) and the median WHO performance status was 1 (range 0–1). Baseline patient and disease characteristics are shown in . All patients had HER2-negative tumors. At the cutoff date, May 2016, all 34 enrolled patients had discontinued from DOS: 16 patients (47%) completed the planned number of cycles of DOS; eight patients (24%) with locally advanced disease had resection; seven patients (21%) discontinued due to disease progression; one (3%) patient discontinued due to an adverse event; and two due to other reasons. A total of 18 patients received S-1 maintenance therapy every three weeks. After resection, three patients continued with four cycles of S-1 maintenance therapy and 15 of 16 patients who completed the planned number of DOS received a median of five (2–9) cycles of S-1 maintenance therapy.
Table 2. Baseline characteristics for all patients treated with DOS.
DOS2w
Three patients were treated at dose level 1A, 2A and 3A, respectively without any sign of DLT (). At dose level 4A, one third of patients developed DLT (febrile neutropenia grade 4) and three more patients were enrolled. No more patients had DLT after first cycle but additionally, one patient had febrile neutropenia after cycle 3; two patients received pegfilgastrim from the third cycle, and two patients had dose reduction from the fourth cycle due to neutropenia. Therefore, dose level 4A could not be recommended as standard therapy without prophylactic pegfilgastrim and three more patients were included on dose level 3A to confirm tolerability without prophylactic granulocyte-colony stimulating factor (G-CSF). Three further patients at dose level 3A confirmed tolerability of DOS2w. The RD of triplet DOS every two weeks in Caucasian patients were: docetaxel 40 mg/m2 on Day 1, O 70 mg/m2 on Day 1 and S-1 35 mg/m2 twice daily on Days 1–7.
A total of 9/18 (50%) patients () achieved a partial response (PR); 4/6 patients with locally advanced disease had sufficient shrinkage of the primary tumor to allow R0 resection after a median of four cycles of DOS2w; one patient at dose level 4A achieved a pathological complete response (pCR). Three patients had progressive disease before nine cycles of DOS. The mPFS was 8.5 months (95% CI 6.8–16.1 months) and the mOS was 14.4 months (95% CI 12.4–17.7; ).
Figure 1. Overall survival. Kaplan-Meier estimated overall survival by initial tumor stage. All locally advanced were T4 tumors.
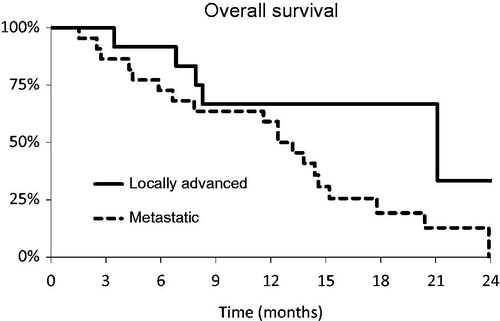
Table 3. Efficacy data for DOS phase I (n = 34).
DOS3w
None of the three patients at dose level 1B and 2B developed a DLT (). At dose level 3B, 2/4 patients developed DLT (febrile neutropenia and febrile neutropenia with diarrhea); the fifth patient received prophylactic pegfilgastrim and further inclusion at dose level 3B was therefore halted. Therefore at least three more patients were scheduled to be included at dose level 2B, and five further patients confirmed the tolerability of DOS3w at dose level 2B. For standard therapy and for further development the RD of DOS3w in Caucasian patients were: docetaxel 50 mg/m2 on Day 1, O 100 mg/m2 on Day 1 and S-1 25 mg/m2 twice daily on Days 1–14.
A total of 56% (9/16) evaluable patients achieved a PR (); mPFS was 9.1 months (95% CI 5.7–12.4 months) and the mOS was 11.6 months (95% CI 6.6–20.4) (). Four of six patients with locally advanced disease had sufficient shrinkage of the primary to allow an R0 resection after a median of three cycles of DOS3w. Four patients had progressive disease before the sixth cycle of DOS.
For the whole group of 34 patients, the ORR was 56% (19/34 patients), mPFS was 9.1 months (95% CI 6.8–12.4 months) and mOS was 13.8 months (95% CI 11.6–17.8 months). Eight of 12 patients with locally advanced gastric cancer (LAGC) had resection, mOS for patients with LAGC was 21.1 months (95% CI 8.3–27.7 months), six patients are still alive. Patients with M1 disease had mOS of 13.2 months (95% CI 7.8–14.6 months), three patients are still alive.
Exposure
The median number of DOS2w and DOS3w was eight (range 2–9) and six (1–6), respectively. Patients received a total of 213 cycles of DOS of which 48 (23%) were dose reduced (). No patients at dose level 1A, 2A and 1B had a dose reduction. The median actual dose intensities were close to the planned dose intensities, with median relative dose intensities (ratio of actual dose intensity to planned dose intensity) of 0.9 or higher.
Table 4. Dose intensity.
Safety
For all patients, across all cycles, the most frequently reported adverse events of any grade were: fatigue (88%), nausea (71%), neuropathy (68%), and diarrhea (56%) (). Severe non-hematological adverse events were reported for seven patients (21%), the most common of which were: diarrhea (9%), fatigue (6%), nausea (6%), vomiting (6%) and neuropathy (3%). Neutropenia was observed in 21 patients (62%), and was grade 3 or 4 in severity 16 patients (47%). Febrile neutropenia was seen in seven patients (21%). No toxicity-related deaths were observed.
Table 5. Adverse events (all cycles).
A total of 21 serious adverse events (SAEs) were observed in 17 patients; seven and 10 patients developed a SAE during or after DOS2w and DOS3w, respectively. The most frequent reasons were febrile neutropenia (n = 6) and infection (n = 4). Two patients developed deep-vein thrombosis/pulmonary embolism and two patients were hospitalized for blood transfusion due to bleeding from the primary tumor. Two patients were hospitalized due to nausea and/or vomiting requiring re-hydration (and one of those developed febrile neutropenia during hospitalization, which was already registered as SAE).
One patient with preexisting cardiac and vascular disease developed myocardial infarction after the second cycle DOS2w, stopped DOS and received palliative radiotherapy. The patient had symptomatic relief after the first cycle of DOS, and due to clinical progression three months after RT, DOS2w was reintroduced without cardiac symptoms. Presently, the patient has received three months of DOS without cardiac symptoms with no sign of progressive disease.
Discussion
In this study, we have established RDs for DOS2w and DOS3w for further development in Caucasian patients. Doublet chemotherapy with fluropyrimidine and C is the backbone of treatment for aGEA. Triple regimens with docetaxel have been shown to be more effective than CF regimens. In the V325 trial [Citation4], all efficacy parameters significantly favored DCF over CF alone, with a higher response rate (RR) (37% vs. 25%), longer PFS (5.6 vs. 3.7 months) and longer mOS (9.2 vs. 8.6 months). Even though DCF resulted in an increased risk of severe adverse events, especially febrile neutropenia and/or neutropenic infection (29% vs. 12%) and diarrhea (19% vs. 8%), preservation of quality of life and clinical benefit favored DCF over CF [Citation14,Citation15]. Several other studies and meta-analysis have confirmed the efficacy of taxanes [Citation1,Citation16–18] and a taxane-containing triple combination is presently the most promising regimen, but unfortunately the most optimal schedule is not established: therapy every second or third week; O or C; infusion of 5-FU or oral alternatives and dose of docetaxel are all important topics still to be solved. However, based on data from randomized phase II studies [Citation16,Citation18,Citation19], a regimen administered every two weeks is preferable based on efficacy and tolerability, especially due to lower gastrointestinal toxicity.
Substitution of C with O and infusion of 5-FU with X eases the burden of administration with at least comparable efficacy [Citation3].
The JCOG 9912 study established monotherapy with S-1 as the standard of care in Asian patients with aGEA [Citation20]. Subsequently, the SPIRITS trial compared S-1 with S-1 plus C and established S-1 plus C as the new Japanese standard chemotherapy regimen [Citation21]. The START trial showed that docetaxel also increases the efficacy of S-1 over monotherapy alone [Citation8]. Yamada et al. also found that the combination of S-1 plus O was equally effective but less toxic than C [Citation7]. An ongoing Japanese study (JCOG 1013) is currently comparing the efficacy of triplet docetaxel and cisplatin with doublet C with an estimated enrollment of 740 patients.
In a prior phase I dose-finding trial [Citation12] in chemo-naïve patients with aGEA, we evaluated the combination of X with docetaxel plus O (DOX) with the aim of finding an easily administered, well tolerated outpatient regimen that maintained the efficacy of DCF seen in the V325 trial [Citation4]. In V325 full-dose docetaxel (docetaxel 75 mg/m2) was added to C and 5-FU. We initially assumed that it might be possible to combine X with full-dose docetaxel (75 mg/m2) and full-dose O (130 mg/m2). However, we saw substantial hematological toxicity even before reaching the anticipated dose level, and in order to maintain treatment schedule without undue dose reductions and toxicity, we recommended docetaxel 51 mg/m2 and O 100 mg/m2 with X if administered without prophylactic G-CSF [Citation7]. These recommendations are in line with Asian phase I and II studies that recommend docetaxel 50–52 mg/m2 Day 1, O 100–105 mg/m2 Day 1, and S-1 80 mg/m2 Days 1–14 every three weeks [Citation9–11]. A recent meta-analysis comparing S-1-based with non-S-1-based chemotherapy in patients with aGEA showed that S-1-based therapy was associated with higher RR [Citation22]. When comparing S-1 with X no difference on efficacy was seen, although a lower rate of grade 3–4 neutropenia and hand-foot syndrome was observed. In the Western subgroup, S-1-based therapy showed significantly lower rates of febrile neutropenia, treatment-related deaths, mucositis and diarrhea [Citation22]. For all these reasons we thought that DOS might be the optimal combination for triplet chemotherapy in Caucasian patients. We therefore planned this study to examine the combination of DOS with the aim of providing similar efficacy to DCF with a regimen that is easier to administer and hopefully less toxic. In Europe, a triplet regimen administrated every three weeks has been one of several standards for many years. However, as stated above we thought that a DOS2w would be at least as promising as DOS3w. Therefore, we designed the present study as two parallel running phase 1/2a trials.
The present phase 1/2a study generated RDs of DOS2w or DOS3w. We found no new safety concerns apparent for the DOS combination. As might be expected, the most frequently reported severe adverse events were hematological toxicities, especially neutropenia, with the DLTs being febrile neutropenia and/or infection. Non-hematological toxicities were very infrequent at the RD levels (3A and 2B). The RD of S-1 is in line with the S-1 dose commonly recommended for Caucasian patients [Citation5,Citation23,Citation24], but lower than doses used in East Asian patients; which we believe is due to more effective bioactivation of tegafur to 5-FU caused by higher activity of CYP2A6 in Caucasian patients [Citation25].
In summary, the promising activity of DOS with manageable toxicity with median actual dose intensities of 0.9 or higher supports further clinical development of this combination. Our study confirms that a S-1 dose of 35 mg/m2 given twice daily on Days 1–7 combined with O 70 mg/m2 and docetaxel 40 mg/m2 every two weeks or a S-1 dose of 25 mg/m2 given twice daily on Days 1–14 combined with O 100 mg/m2 and docetaxel 50 mg/m2 every three weeks are appropriate first-line regimens for future trials in Caucasian patients with aGEA.
Acknowledgments
We thank all the patients, their families, and the medical staff.
Disclosure statement
Per Pfeiffer has received a lecturer’s fee from Taiho. Camilla Qvortrup, Merete Krogh, Katrine Schoennemann, Lene W. Vestermark, Helle A. Jensen, Jon K. Bjerregaard declare that they have no conflict of interest. Taiho Pharmaceutical Co. Ltd and Nordic Group BV was not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Additional information
Funding
References
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010:CD004064. doi: 10.1002/14651858.CD004064.pub3.
- Lordick F, Allum W, Carneiro F, et al. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. 2014;40:692–700.
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997.
- Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553.
- Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer. 2013;49:3616–3624.
- Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148.
- Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319–328.
- Park I, Ryu MH, Choi YH, et al. A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2013;72:815–823.
- Zang DY, Yang DH, Kim MJ, et al. Dose-finding study of docetaxel, oxaliplatin, and S-1 for patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2009;64:877–883.
- Kim HS, Ryu MH, Zang DY, et al. Phase II study of docetaxel, oxaliplatin, and S-1 therapy in patients with metastatic gastric cancer. Gastric Cancer. 2016;19:579–585.
- Andersen M, Schonnemann KR, Yilmaz M, et al. Phase I study of docetaxel, oxaliplatin and capecitabine (TEX) as first line therapy to patients with advanced gastro-oesophageal cancer. Acta Oncol. 2010;49:1246–1252.
- Schonnemann KR, Yilmaz M, Andersen M, et al. New recommendation of doses in an ongoing phase II study of docetaxel, oxaliplatin and capecitabine as first line therapy in advanced gastro-oesophageal cancer. Acta Oncol. 2011;50:151–152.
- Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205–3209.
- Ajani JA, Moiseyenko VM, Tjulandin S, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3210–3216.
- Van Cutsem E, Boni C, Tabernero J, et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann Oncol. 2015;26:149–156.
- Wang J, Xu R, Li J, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19:234–244.
- Shah MA, Janjigian YY, Stoller R, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US gastric cancer consortium. J Clin Oncol. 2015;33:3874–3879.
- Al-Batran SE, Pauligk C, Homann N, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer. 2013;49:835–842.
- Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069.
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221.
- Ter Veer E, Mohammad NH, Lodder P, et al. The efficacy and safety of S-1-based regimens in the first-line treatment of advanced gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2016;19:696–712.
- Winther SB, Zubcevic K, Qvortrup C, et al. Experience with S-1 in older Caucasian patients with metastatic colorectal cancer (mCRC): findings from an observational chart review. Acta Oncol. 2016;55:881–885.
- Moehler M, Mahlberg R, Heinemann V, et al. Phase I study of orally administered S-1 in combination with epirubicin and oxaliplatin in patients with advanced solid tumors and chemotherapy-naive advanced or metastatic esophagogastric cancer. Gastric Cancer. 2016. doi: 10.1007/s10120-016-0618-0.
- Chuah B, Goh BC, Lee SC, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci. 2011;102:478–483.
