To the Editor,
Brain metastases of melanoma may occur in up to 50–75% of patients with stage IV disease [Citation1], and two-thirds of them are diagnosed prior to death [Citation2,Citation3]. The prognosis is poor, irrespective of treatment including whole brain radiotherapy (WBRT), with or without surgery or stereotactic radiosurgery; the median survival ranges from 2 to 6 months [Citation4–7]. Therefore, the intervention should be as short as possible. As melanomas are assumed to have a low radiosensitivity to small fractions [Citation8], we have retrospectively compared an accelerated, hypofractionated schedule administered over five days at one center with a more standard schedule at another nearby center using fractionations in common use.
Methods
Patients with brain metastases diagnosed by computed tomography (CT) scan and treated with WBRT were included from consecutive patient charts at the radiotherapy departments of the West Swedish Health Care region (Center A), and the South Swedish Health Care region (Center B). All had histologically verified malignant melanoma in the past, with or without extracranial manifestations.
Between 1993 and 2003, 50 patients from Center A were routinely treated by X-rays of 4–6 MV using lateral opposed portals including the whole brain with a mid-dose of 3.3 Gy twice daily, with 6–8 hours in between, for five consecutive days. In Center B, 48 patients were recruited between 1985 and 1995 using similar radiation conditions, but with fractionation: 3.0 Gy daily over 12 days to 30 Gy (n = 33), 3.0 Gy daily over 16 days to 36 Gy (n = 8), 4 Gy daily over five days to 20 Gy (n = 5), and 5 Gy daily over five days to 25 Gy (n = 2).
Results
Patient characteristics are summarized in . Survival was registered from start of radiotherapy (RT) (). The two groups were similar in terms of number of patients, age, gender, and latency between primary tumor and brain metastases. Center A had more patients with multiple metastases and considerably more documented extracerebral metastases than Center B, 92% and 52%, respectively. At Center A, the group without surgery prior to RT had 10 days’ longer delay between diagnosis and treatment (). Overall survival did not differ significantly between the two groups (). The 30-day post-irradiation mortality was similar: five patients at each center, none of them with single lesions.
Figure 1. Kaplan-Meier plot of overall survival from start of radiotherapy (no significant between-cohort difference).
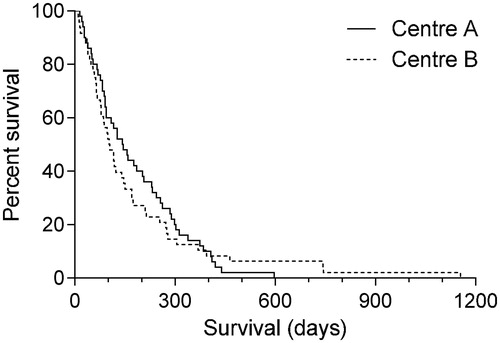
Table 1. Patient characteristics.
Table 2. Survival.
Discussion
RT dose and fractionation should be based on the radiobiology of the lesion as well as the surrounding normal tissue, together with the goal of the treatment, here palliative. Survival curves of melanoma cell lines were reported to have large ‘shoulders’ indicating the use of larger fractions in RT of melanomas [Citation9]. To these early data both a wide diversity of α/ß ratios in vitro has later been reported [Citation10] and clinical evidence supporting the use of larger fractions (i.e. low α/ß ratios) [Citation8]. Only one randomized study has been performed, comparing 4 × 8 Gy over 21 days with 20 × 2.5 Gy over 27 days, with no difference in outcome [Citation11]. Khan et al. concluded that recent studies suggest a significant role for radiation therapy in melanoma treatment under certain clinical circumstances, despite the relatively radio-insensitive tumor type [Citation12]. With improved molecular characterization, selective interventions, for example those with concomitant B-RAF inhibition might improve the outcome [Citation13].
We considered it of interest to compare the outcome of different fractionation schedules in two nearby and similar centers. lists the fractionation schedules with the biologically effective dose (BED) and the equivalent total dose in 2 Gy fractions (EQD2) [Citation14], for two α/ß ratios. The brain is assumed to have a ratio of approximately 3. As discussed above, the lesions to be treated may have an α/ß ratio similar to or even less than that of the brain, but also may be high as for acute responding tissues (α/ß=10). The values calculated for the twice daily fractions include a correction of 10% for incomplete repair, for instance a dose per fraction of 3.63 was used in the calculations [Citation15,Citation16].
Table 3. Fractionation schedules.
Our data represent the consecutive series of patients treated with whole brain irradiation of malignant melanoma. At Center B a few patients were treated with a short-term fractionation over one week, resulting in a lower BED. Subanalysis showed extremely short survival compared to the rest of the material, but these patients were probably selected for a shorter treatment due to a more advanced disease.
In 2008, McWilliams et al. [Citation2] summarized several previous studies of different fractionation schedules for WBRT, and found no indication that larger doses per fraction could overcome the relative radioresistance of melanoma. A retrospective study by Rades et al. suggested that patients with brain metastases from melanoma treated with WBRT alone may benefit from an escalation of the total dose [Citation17].
Fractions as large as 3.3 Gy twice daily to the brain for five consecutive days is unusual, and has not been evaluated. This schedule gives a large total dose in a short period of time, and is a compromise using an intermediate fraction size delivered over five days, comparable to the regimen (3.0–3.5 Gy bid × V) [Citation18].
The patients’ characteristics were similar between the groups and also similar to those seen in larger studies [Citation19,Citation20]. The difference in presence of extracerebral metastases may be explained by examination with CT scan of the chest and abdomen was less frequent in 1985–1995 (Center B data gathered) than in 1993–2003 (Center A data gathered). In larger studies, the presence of extracerebral metastases is reported at 46–94% [Citation6,Citation20].
From this retrospective study it was not possible to find the pre-treatment Karnofsky score and RPA classification [Citation21], nor the post-treatment toxicity, quality of life, or objective responses; survival was the only measurable outcome. Our results with a median survival of 4.7 and 3.4 months, respectively, are comparable to published data [Citation4–7,Citation20].
The QUANTEC report from 2009 summarized late side effects of RT to the brain [Citation22], showing that for twice daily fractionation a steep increase in radiation necrosis of the brain, 1–2 years after RT, appears to occur at a BED of >80 Gy (α/ß 3 Gy), and fractions of <2 Gy. The BED (α/ß 3 Gy) for the schedule 3.3 Gy bid × V is 80.2 Gy. Long-term survivors were scarce at both centers up to 20 months and up to 38 months. No post mortem examinations were performed to elucidate tumor status, or to examine any late normal tissue damage which may have differed between the two groups. Acute fatal complications following the short-term schedule do not seem to have been more frequent, as the 30-day mortality was similar.
It has been concluded that poor-prognosis patients may be more optimally managed with steroids in a prospective symptom response study in patients with brain metastases from different primary tumors [Citation23]. It is important to identify the patients with poor prognosis, using recursive partitioning analyses [Citation4,Citation19]. Although we lacked the information to perform a recursive partitioning classification, it is questionable whether our patients with five or more lesions benefited from RT, irrespective of fractionation.
In conclusion, a presently common fractionation schedule for brain metastases is 20 Gy in five daily fractions irrespective of metastatic origin. Implementing a twice daily fractionation may be impractical at most departments but the favorable five-day treatment course may best be accomplished by using a 5 × 5 Gy schedule often used in the pelvic volume, thus achieving a significantly higher dose than the 4 × 5 Gy ().
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Patel JK, Didolkar MS, Pickren JW, et al. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810.
- McWilliams RR, Rao RD, Buckner JC, et al. Melanoma-induced brain metastases. Expert Rev Anticancer Ther. 2008;8:743–755.
- Hong A, Fogarty G, Izard MA. The role of radiation therapy in the management of metastatic melanoma in the brain. Int J Surg Oncol. 2012;2012:294735.
- Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293–1300.
- Samlowski WE, Watson GA, Wang M, et al. Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer. 2007;109:1855–1862.
- Stone A, Cooper J, Koenig KL, et al. A comparison of survival rates for treatment of melanoma metastatic to the brain. Cancer Invest. 2004;22:492–497.
- Ziegler JC, Cooper JS. Brain metastases from malignant melanoma: conventional vs. high-dose-per-fraction radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12:1839–1842.
- Bentzen SM, Overgaard J, Thames HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989;16:169–182.
- Dewey DL. The radiosensitivity of melanoma cells in culture. Br J Radiol. 1971;44:816–817.
- Rofstad EK. Radiation biology of malignant melanoma. Acta Radiol Oncol. 1986;25:1–10.
- Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991;20:429–432.
- Khan N, Khan MK, Almasan A, et al. The evolving role of radiation therapy in the management of malignant melanoma. Int J Radiat Oncol Biol Phys. 2011;80:645–654.
- Sambade MJ, Peters EC, Thomas NE, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394–399.
- Joiner M, Bentzen SM, Basic clinical radiobiology: Fractionation: The linear quadratic approach. 4th ed. London: Hodder Arnold; 2009.
- Nilsson P, Thames HD, Joiner MC. A generalized formulation of the 'incomplete-repair' model for cell survival and tissue response to fractionated low dose-rate irradiation. Int J Radiat Biol. 1990;57:127–142.
- Nyman J, Turesson I. Does the interval between fractions matter in the range of 4-8 h in radiotherapy? A study of acute and late human skin reactions. Radiother Oncol. 1995;34:171–178.
- Rades D, Heisterkamp C, Huttenlocher S, et al. Dose escalation of whole-brain radiotherapy for brain metastases from melanoma. Int J Radiat Oncol Biol Phys. 2010;77:537–541.
- Choi KN, Withers HR, Rotman M. Metastatic melanoma in brain. Rapid treatment or large dose fractions. Cancer. 1985;56:10–15.
- Meier S, Baumert BG, Maier T, et al. Survival and prognostic factors in patients with brain metastases from malignant melanoma. Onkologie. 2004;27:145–149.
- Sampson JH, Carter JH, Jr., Friedman AH, et al. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20.
- Morris SL, Low SH, A'hern RP, et al. A prognostic index that predicts outcome following palliative whole brain radiotherapy for patients with metastatic malignant melanoma. Br J Cancer. 2004;91:829–833.
- Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–S27.
- Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer. 2002;38:487–496.
