Abstract
Background: Re-irradiation (Re-RT) is offered widely in clinical routine, and has been established as a key element in the treatment of recurrent gliomas. At our center, generally re-resection is performed widely by an experienced neurosurgical team. Thus, Re-RT mostly offered to patients with macroscopic residuals or irresectable lesions, is applied later compared to other centers. Therefore, we sought to validate the Combs Prognostic Score developed in 2012 using our independent patient cohort.
Patients and methods: We included 199 patients treated from 2002 until April 2016 for recurrent glioma at the Department of Radiation Oncology at the Klinikum Rechts der Isar, Munich. Different concepts of Re-RT were applied.
Results: Median follow-up after Re-RT was 2.5 months. Median overall survival (OS) after Re-RT was 7.9 months for WHO IV gliomas, 11.3 months for WHO III gliomas, and 13.6 months for low-grade gliomas (WHO I/II). Univariate analyses confirmed the prognostic factors primary histology (p = 0.001), age (p = 0.002), and time between primary radiotherapy and Re-RT (p < 0.001). We also tested Karnofsky Performance Score (KPS), gender, and neurological symptoms before Re-RT as well as planning target volume and found only KPS also significant at p < 0.001. Comparing the prognostic score groups, the outcome was highly statistically significant at p < 0.001.
Conclusion: In our analysis, we validated the Combs Prognostic Score. Validation in this independent large patient cohort confirms the significance of the score for glioma recurrences. Thus, the role of the Combs Prognostic Score might be an essential component of future clinical decision making and patient stratification.
The role of re-irradiation (Re-RT) is still controversially discussed for patients with recurrent gliomas. Although decades ago a second course of radiotherapy (RT) was not possible due to the risk of treatment-related side effects, modern RT offers precise dose deposition and steep dose gradients to normal tissue [Citation1]. Re-RT is now offered widely in clinical routine, and has been established as a key element in the treatment of recurrent gliomas [Citation2,Citation3]. Different dosing regimens are available, depending on institutional preferences, applied technique, size, and location of the lesion, as well as the time range between primary and secondary RT. The largest series have irradiated with a total dose of 36 Gy in 2 Gy single fractions [Citation4]. Often, it is discussed whether the dose is sufficient, and extensive literature research confirms that further dose escalation might be possible [Citation5]. Smaller series have increased the dose, and some facilities use hypofractionation RT concepts. Comparing available data, progression-free survival ranges from 3 to 11 months [Citation6,Citation7].
The patient population of recurrent gliomas is heterogeneous, including various histologies, time intervals between primary and secondary RT, different target volume concepts, and different time points during the course of the disease. While some centers are reluctant to offer a second course of RT in general, others offer recurrence resection more widely and Re-RT is postponed. To address the issue of heterogeneity, several approaches try to take patient and tumor characteristic into account for decision making. Our group has previously developed a prognostic score, which Combs and colleagues published in 2012, to predict survival outcome after Re-RT of recurrent glioma [Citation8].
Comparative analyses with other scoring approaches have revealed conflicting results, with a different value of each score [Citation9,Citation10]. However, the choice of Re-RT is made on a case-by-case basis, and solid data or scoring schemes including patient-related data, molecular data and/or other individual information might be useful in the future to stratify patient subgroups.
At the Technical University of Munich (TUM) recurrent gliomas are seen in an interdisciplinary setting. Due to the high throughput at the Neurooncology center, a large number of patients with glioma recurrences are seen. Thus, an independent population for validation of the ‘Combs Prognostic Score’ [Citation8] is available to confirm the clinical relevance in a major Neurooncology center.
Patients and methods
To validate the Combs Prognostic Score in a further institution, we included 199 patients treated consecutively from 2002 until April 2016 for recurrent glioma at the Department of Radiation Oncology at the Klinikum Rechts der Isar, Munich. Patient data were collected prospectively in the institutional database. The local ethics committee of the TUM Medical Faculty approved the study with vote number 408/14.
Median age was 56 years (range 22–79 years). For patients characteristic see . Primary RT was applied in our center or externally with a median dose of 60 Gy (range 50–64 Gy) in single fractions of 1.8 or 2.0 Gy. The treatment decision for Re-RT was based on interdisciplinary tumor boards. Concomitant chemotherapy with temozoloide (TMZ) was performed when indicated. No other chemotherapeutic treatments or molecular targeted substances, for instance bevacizumab, were applied parallel to RT.
Table 1. Patients’ characteristics.
Treatment was performed in a stereotactic setup based on computed tomography (CT), magnetic resonance imaging (MRI) (T1) and amino acid positron emission tomography (PET) when available. The clinical treatment volume (CTV) included the macroscopic tumor and a safety margin of about 5 mm; the planning target volume (PTV) included an additional margin of 1–2 mm. Median PTV volume was 62.0 ml (range 0.4–480.6 ml). Different concepts of Re-RT were applied depending on volume and location of the lesion, the time between primary RT and Re-RT, and the treating physician’s preference. Thus, hypofractionated RT, radiosurgery, and normal fractionated RT were applied and included in this evaluation (). If re-resection was performed after initial primary RT, new histology findings were available. Therefore, lists histology at recurrence as well.
All patients were enrolled into a tight follow-up regimen with 2–3 month checkups, including contrast-enhanced MRI and clinical assessment. Additional examinations were scheduled as clinically needed. All decisions about further therapies were made on an interdisciplinary basis.
Statistical calculations were performed using SPSS Statistics v23 (IBM, USA). For all patients, overall survival (OS) was calculated from the first day of Re-RT until death or last follow-up. We analyzed the following prognostic factors, according to the previously published data [Citation8]: primary histology, the time between primary RT and Re-RT, and age. The scoring scheme, according to the Combs Prognostic Score is summarized in . The sum of the values results in the prognostic score. We expanded the analyses and further included: Karnofsky Performance Score (KPS), neurological symptoms, gender, PTV, and dose group. Survival and multivariate analyses were based on the Cox regression method. A p value ≤0.05 was considered as statistically significant.
Table 2. Scoring scheme to calculate the prognostic score according to the previously published work by Combs et al. [Citation8].
Results
Outcome
The median follow-up after Re-RT was 2.5 months (range 0–91.1 months, including foreign patients who were lost to follow-up and patients who immediately died after or during Re-RT). According to primary histology, median OS after Re-RT was 7.9 months (range 1–100 months) for WHO IV gliomas, 11.3 months (range 1–55 months) for WHO III gliomas and 14.3 months (range 1–57 months) for low-grade gliomas (WHO I/II).
Univariate analyses confirmed the prognostic factors primary histology (p ≤ 0.001), age (<50 years vs. ≥ 50 years, p = 0.002) and time between primary RT and Re-RT (>12 months vs. ≤12 months, p < 0.001). We also tested KPS (≥80% vs. <80%, p < 0.001), gender (p = 0.8), neurological symptoms before Re-RT (p = 0.3) as well as PTV volume (≥47 ml vs. <47 ml, p = 0.1) and found only KPS also significant (, supplementary material).
Table 3. p Values for univariate and multivariate analysis on overall survival (using log-rank test).
Examining the different RT treatment regimes, we found a significant difference in OS between the treatment dose groups (p = 0.038). However, median survival varied from 7.6 months in Group A (mainly 36 à 2 Gy), 9.6 months in Group B (30 à 5 Gy), 6.7 months in Group C (36 à 3 Gy) and 11.3 months in Group D (mainly 46 à 2 Gy).
In the multivariate analyses histology (p = 0.003), the time between RT and Re-RT (p = 0.05), KPS (p < 0.001) and dose group (p = 0.018) remained significant.
Validation of the Combs Prognostic Score
We subdivided all patients into different scoring groups based on established prognostic factors histology, age, and time between primary RT and Re-RT. According to these subgroups, the Combs Prognostic Score was calculated as described previously [Citation8]. In our patient cohort, the distribution was: n = 14 (7.0%) as excellent (0 points), n = 30 (15.1%) as good (1 point), n = 41 (20.6%) as moderate (2 points), n = 66 (33.2%) as poor (3 points) and n = 48 (24.1%) as very poor (4 points) (). We compared the survival (OS) according to these scoring groups, which showed a highly significant correlation with p < 0.001 ().
Figure 1. Overall survival after Re-irradiation according to the Combs Prognostic Score (p < 0.001*).
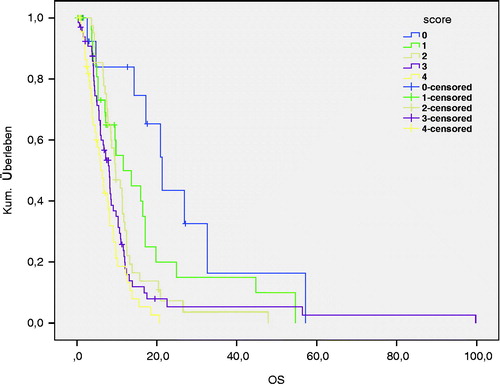
Table 4. Combs Prognostic Score: median overall survival and life table.
Discussion
In our analysis, we validated the Combs Prognostic Score [Citation8] by means of data from 199 glioma patients. All patients were consecutively treated with Re-RT in a single center and followed prospectively in an institutional database. Therefore, this dataset represents one of the largest cohorts from any single center offering a homogeneous quality and strategy for Re-RT to validate the score. Statistical analyses showed a sound confirmation of the Combs Prognostic Score with a significance level of p < 0.001.
Treatment of glioma recurrences remains a controversial topic and depends on the specialization of the treatment center [Citation11,Citation12]. Many centers favor Re-RT. Since the introduction into clinical routine indication criteria changed: initially, due to the fear of side effects, the inclusion criteria were strict and restrictive and included only first recurrences, longer times between the previous RT, as well as small treatment volumes. This was mainly due to published data demonstrating limited efficacy and a potential for treatment-related side effects [Citation13]. The availability of highly precise RT in clinical routine changed the paradigm: Re-RT has been validated as a safe and effective treatment alternative. Different dosing regimens have been established, that depend on the volume of the lesion, the time between primary RT and recurrent RT, overall patient performance status, and other patient-individual characteristics. To date, no clear evidence supports either normal fractionated or hypofractionated concepts. Keeping in mind that most recurrences develop within the primary RT field and concomitant systemic therapies potentially modify the radiation-sensitivity of normal tissue, many groups favor normal fractionated, or slightly hypofractionated (3 Gy single dose) approaches. Previously, we could establish the regimen of 36 Gy in 2 Gy single fractions, however, subsequent calculations hypothesized that further dose-intensification might be possible [Citation4,Citation8].
Besides Re-RT, the role of re-resection is an additional point of controversy. Although there is some evidence on the benefit of surgery for recurrent gliomas, no randomized data confirm this hypothesis, and thus the indication is generally based on the institution’s experience and on patient-individual factors.
To shed light on the role of Re-RT, we previously developed a prognostic score [Citation8]. This score aimed to be a useful tool to determine the range of benefit in patients with recurrent gliomas. Several groups thereafter recognized the potential value of such a scoring system in the clinical setting. In 2012, Scholtyssek et al. [Citation10] analyzed 64 patients with high-grade gliomas and could not confirm the significant difference in survival of the different scoring groups. Niyazi et al. [Citation9] validated the Combs Prognostic Score on 30 patients with high- and low-grade tumors in 2014, and also found no significant influence on OS (p = 0.664). All of these evaluations were either based on small, single-center cohorts, or on multi-institutional databases. While small cohorts rarely have sufficient power, pooled datasets are limited by heterogeneities in data collection, treatment algorithms, or other factors. This easily explains why most approaches to validate the Combs Prognostic Score failed. However, in the present TUM cohort, a very large homogeneous dataset of 199 patients from a single institution is used for independent validation. Based on the established prognostic factors, the score is demonstrated to be highly significant. It yet remains to be evaluated how such a scoring system can be included in clinical decision making, for example real-time use within interdisciplinary tumor boards.
Generally, the main criticism, as well as a limitation of our score and similar approaches, may depend on the input data, which of course have the main influence on the value of such a score. Other arguments might be the heterogeneity of data included, the molecular variability behind any tumor type is often not sub-classified in a simple clinical database, as well as the model used for calculating the score. Today, modern databases offer all clinical data including imaging, molecular data as well as all relevant clinical information [Citation14]. Automatic generation of a prospective database-driven tool for decision making readily available for clinical routine seems a logical consequence offering the possibility to deliver an online scoring system for each tumor type; this information is then readily available for interdisciplinary decision making such as tumor boards. In this context, the real value of any prognostic score can be exploited.
Conclusion
The Combs Prognostics Score is a significant and useful tool to predict the overall effect of Re-RT in patients with recurrence gliomas. Validation in a very large independent cohort confirmed this hypothesis. In the future, the score could serve as a stratification factor both in prospective clinical trials and in clinical routine.
IONC_1276621_Supplemental_figures.zip
Download Zip (107 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Combs DSE, Schulz-Ertner D, Herfarth KK, et al. Fortschritte in der Radioonkologie. Chirurg. 2006;77:1126–1132.
- Combs SE, Debus J, Schulz-Ertner D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer. 2007;7:167.
- Dhermain F, de Crevoisier R, Parker F, et al. [Role of radiotherapy in recurrent gliomas]. Bull Cancer. 2004;91:883–889.
- Combs SE, Thilmann C, Edler L, et al. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869.
- Amichetti M, Amelio D. A review of the role of re-irradiation in recurrent high-grade glioma (HGG). Cancers. 2011;3:4061–4089.
- Torcuator RG, Thind R, Patel M, et al. The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol. 2009;97:401–407.
- Wurm RE, Kuczer DA, Schlenger L, et al. Hypofractionated stereotactic radiotherapy combined with topotecan in recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 2006;66:S26–S32.
- Combs SE, Edler L, Rausch R, et al. Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol. 2012;52:147–152.
- Niyazi M, Flieger M, Ganswindt U, et al. Validation of the prognostic Heidelberg re-irradiation score in an independent mono-institutional patient cohort. Radiat Oncol. 2014;9:128–126.
- Scholtyssek F, Zwiener I, Schlamann A, et al. Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol. 2013;8:161.
- Palmer JD, Siglin J, Yamoah K, et al. Re-resection for recurrent high-grade glioma in the setting of re-irradiation: more is not always better. J Neurooncol. 2015;124:215–221.
- Roy S, Lahiri D, Maji T, et al. Recurrent glioblastoma: where we stand. South Asian J Cancer. 2015;4:163–173.
- VanderSpek L, Fisher B, Bauman G, et al. 3D conformal radiotherapy and cisplatin for recurrent malignant glioma. Can J Neurol Sci. 2008;35:57–64.
- Kessel KA, Bohn C, Engelmann U, et al. Five-year experience with setup and implementation of an integrated database system for clinical documentation and research. Comput Methods Programs Biomed. 2014;114:206–217.
