Abstract
Purpose: To report 5-year outcomes of a prospective trial of image-guided accelerated hypofractionated proton therapy (AHPT) for prostate cancer.
Patients and methods: 215 prostate cancer patients accrued to a prospective institutional review board-approved trial of 70Gy(RBE) in 28 fractions for low-risk disease (n = 120) and 72.5Gy(RBE) in 29 fractions for intermediate-risk disease (n = 95). This trial excluded patients with prostate volumes of ≥60 cm3 or International Prostate Symptom Scores (IPSS) of ≥15, patients on anticoagulants or alpha-blockers, and patients in whom dose-constraint goals for organs at risk (OAR) could not be met. Toxicities were graded prospectively according to Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. This trial can be found on ClinicalTrials.gov (NCT00693238).
Results: Median follow-up was 5.2 years. Five-year rates of freedom from biochemical and clinical disease progression were 95.9%, 98.3%, and 92.7% in the overall group and the low- and intermediate-risk subsets, respectively. Actuarial 5-year rates of late radiation-related CTCAE v3.0 grade 3 or higher gastrointestinal and urologic toxicities were 0.5% and 1.7%, respectively. Median IPSS before treatment and at 4+ years after treatment were 6 and 5 for low-risk patients and 4 and 6 for intermediate-risk patients.
Conclusions: Image-guided AHPT 5-year outcomes show high efficacy and minimal physician-assessed toxicity in selected patients. These results are comparable to the 5-year results of our prospective trials of standard fractionated proton therapy for patients with low-risk and intermediate-risk prostate cancer. Longer follow-up and a larger cohort are necessary to confirm these findings.
Keywords:
Introduction
Interest in accelerated hypofractionated photon radiation therapy in the treatment of prostate cancer has developed with the recent ability to create highly conformal photon-based radiation dose distributions, and several investigators have reported safety and efficacy with various hypofractionation schemes [Citation1–4]. Proton therapy provides more conformal radiation dose distributions than photon-based radiation therapy, but mature data on clinical outcomes with accelerated hypofractionated proton therapy (AHPT) are limited [Citation5]. The purpose of this study is to report the 5-year outcomes of a prospective trial to determine the safety and efficacy of AHPT in prostate cancer.
Materials and methods
The patients
From April 2008 through October 2011, 215 patients enrolled in an institutional review board-approved protocol, PR04, for low-risk (n = 120) and intermediate-risk (n = 95) prostate cancer designed to test the feasibility of an AHPT regimen. Eligibility criteria included biopsy-proven prostate cancer, clinical stage T1–T2b, prostate-specific antigen (PSA) level ≤20 ng/ml, Gleason Score ≤7, and no nodal or distant metastases. This trial can be found on ClinicalTrials.gov (NCT00693238).
Hormonal therapy was not required given the 99% 5-year freedom from biochemical and clinical disease progression rate in our previously published benchmark trial of conventionally fractionated proton treatment of intermediate-risk prostate cancer and patient preference to avoid the side effects and risks associated with androgen deprivation [Citation6]. However, 2 patients received a single 3-month injection of gonadotropin-releasing hormone agonist before proton therapy, and 1 patient had an oral androgen receptor inhibitor. Six of the 7 patients on 5-alpha-reductase inhibitors discontinued them prior to proton therapy, but 1 continued during and after AHPT. Exclusion criteria shared with our prior studies of conventionally fractionated proton therapy for low- and intermediate-risk prostate cancer included the following: prior prostate cancer surgery or local prostate cancer treatment; active inflammatory bowel disease affecting the rectum; and history of proximal urethral stricture. Additional exclusion criteria unique to this AHPT study included the following: International Prostate Symptom Score (IPSS) of ≥15; use of alpha-blockers; diabetes mellitus; prior intrapelvic surgery; current and continuing use of anticoagulation, saw palmetto or immunosuppressants; and inability to meet prespecified dose constraints for organs at risk (OAR) in the treatment planning process. Low- and intermediate-risk patients had a maximum prescribed dose of 70 and 72.5 Gy(RBE) to the planning target volume (PTV), respectively, but could have a dose reduction to a minimum of 67.5 Gy(RBE) if needed to meet OAR constraints. The recommended dose to the proximal seminal vesicles (SV) was 60 Gy(RBE), but a dose reduction to a minimum of 45 Gy(RBE) was allowed to meet OAR constraints. Dose constraints for the bladder and rectum in this study were alpha–beta ratio conversions of constraints used in our previously reported prospective trials of conventionally fractionated proton therapy [Citation6]. A protocol amendment excluded patients with a prostate volume of ≥60 cm3 after 16 April 2009. Required staging included medical history and physical examination, complete blood count, testosterone level, PSA, alkaline phosphatase, computerized tomography and magnetic resonance imaging (MRI) (unless contraindicated) of the pelvis, chest X-ray, and ≥10-core biopsy (12-core preferred) within 6 months prior to study enrollment. The histologic diagnosis was confirmed by a second pathologist from our institution or a reference lab.
PSA values were obtained before and at the end of treatment, at 3-month intervals for 3 years, at 6-month intervals for an additional 2 years, and then annually. PSA progression was defined by the Phoenix criterion (nadir +2 ng/ml). Patients with PSA progression had a digital rectal exam, bone scan and MRI of the pelvis and/or positron emission tomography (PET)–computed tomography (CT) to determine patterns of failure. Post-treatment prostate biopsy was performed only if local recurrence was suspected. Physician-verified toxicities were scored using the Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3.0), weekly during treatment and then at 6-month intervals [Citation7]; serious adverse events were also classified retrospectively according to the 2010 edition of the CTCAE v4.0 to facilitate comparison with other series. IPSS was assessed before and at 6-month intervals after PT. Overall, 203 patients (94.4%) were examined or contacted within 12 months of this analysis or were deceased. The median actual follow-up times (with toxicity and PSA data) were 5.3 years (range, 2.2–7.2) for low-risk patients, 5.1 years (range, 1.8–7.2) for intermediate-risk patients, and 5.2 years (range, 1.8–7.2) overall.
Patient characteristics are provided in . Twenty-nine of the 120 low-risk patients (24.2%) were ‘very low-risk’ according to the National Comprehensive Cancer Network Clinical Practice Guidelines [Citation8]. Twenty-four of the 95 intermediate-risk patients (25.3%) were considered ‘unfavorable’ on the basis of a dominant Gleason pattern of 4, and/or a PSA of ≥15 and <20.
Table 1. Patient characteristics.
Protocol treatment
All 120 low-risk patients received 70 Gy(RBE) in 28 fractions (2.5 Gy[RBE]/fraction). Seventy-four intermediate-risk patients received 72.5 Gy(RBE) in 29 fractions (2.5 Gy[RBE]/fraction) to the prostate and 21 intermediate-risk patients received only 70 Gy(RBE) to the prostate to comply with the OAR constraints. The SV in intermediate-risk patients were excluded from the target volume after 60 Gy(RBE), 57.5 Gy(RBE), 50.8 Gy(RBE) and 45 Gy(RBE) in 90, 2, 1 and 2 patients, respectively, to comply with the OAR dose-constraint goals. A total of 22 additional patients were excluded from the study because of an inability to meet OAR constraint goals.
Treatment simulation, planning, and delivery
The details of treatment simulation, planning, and delivery are the same as those described for previous proton therapy protocols with two exceptions: all patients in the current study had a rectal balloon for prostate stabilization, and the PTV expansion to account for potential set-up errors was 6 mm in the cranial-caudal axis and 4 mm axially, as opposed to 8 and 5 mm, respectively, for the prior protocols [Citation9]. This PTV reduction was made possible by the use of the rectal balloon. The clinical target volume (CTV) for low-risk patients was the prostate and for intermediate-risk patients the prostate and proximal 1.5–2 cm of SVs. Patients were treated with opposed lateral or lateral-oblique fields, 1 field per day with passively scattered protons in a supine position, secured by a custom vacuum-locked bag. Orthogonal kilovoltage imaging with alignment on intra-prostatic fiducial markers was used for daily image-guided treatment delivery.
Normal-tissue dosimetric specifications
The dose-constraint goals for the rectal wall were V45 < 50% and V65 < 30%. Minor and major deviations were defined as V45 of 50% to <60% and ≥60% and/or V65 of 30% to <40% and ≥40%. A secondary rectal dose-constraint goal limited the absolute volume of rectum receiving ≥70 Gy (RBE) ≤10 cm3. There were minor rectum deviations in V70 in 4 low-risk and 25 intermediate-risk patients. There were no major deviations in rectum and rectal wall dose constraints (Supplementary Table S1).
Dose-constraint goals for the bladder wall were V27.5 of <35 cm3, V70 < 13cm3, and V72.5 of <8 cm3. Minor and major deviations were classified as V27.5 of 35 to <45 cm3 and ≥45 cm3 and/or V70 of 13 to <15 cm3 and ≥15 cm3 and/or V72.5 of 8 to <10 cm3 and ≥10 cm3. There were minor bladder wall deviations in V70 in 3 low-risk and 2 intermediate-risk patients and minor deviations in V72.5 in 16 intermediate-risk patients, but no major deviations.
Study design and statistics
Before the design of the current proton study, Lukka et al. reported the results of a randomized x-ray trial of 936 men comparing standard fractionation with 66 Gy in 33 fractions to hypofractionation with 52.5 Gy in 20 fractions in patients with localized prostate cancer [Citation10]. The 466 patients on the hypofractionation arm had a National Cancer Institute of Canada grade 3 + rectal and bladder toxicity risk of 11.4%. The primary objective of the current study was based on the hypothesis that the CTCAE v3.0 grade 3 + rectal and bladder toxicity at 6 months with proton therapy would not exceed 7%, so the primary study endpoint was the cumulative incidence of grade 3 + toxicity at 6 months. Using the exact non-inferiority test for a binary proportion (with a null proportion of 7%, a proportion of 15%, and a negative margin of 0.3%), the power analysis demonstrated that there would be 95% power to test the hypothesis with a 95% confidence limit if a minimum of 165 patients were enrolled and treated on the study. If more than 11% of patients experienced a grade 3 + toxicity, then the hypothesis of non-inferiority would be rejected. With approval of the institutional review board, enrollment continued past the minimum required number to a final sample size of 215.
All statistical computations were performed with SAS and JMP software (SAS Institute, Cary, NC). The Kaplan–Meier product limit method provided estimates of disease progression, survival and freedom from toxicity. A log-rank test statistic assessed the level of statistical significance between strata of selected prognostic factors for these endpoints. All p values below .05 were considered statistically significant.
Results
Toxicity
There were no grade 3 or higher toxicities in the first 6 months of follow-up, confirming the study hypothesis. The actuarial rate of late (after 6 months) grade 3 or higher genitourinary (GU) toxicity at 5 years was 1.7% scored prospectively according to the CTCAE v3.0 (CI, 0.5%–5.5%) and 1.0% scored retrospectively by CTCAE v4.0 criteria (CI, 0.2%–3.7%). There were no grade 4 or 5 GU toxicities. All late grade 3 events were transient, except 1 recent event with insufficient follow-up to make a determination concerning transiency. GU events were not correlated with pretreatment IPSS (<12 versus ≥12; p = .6909). There was no association between prostate size and GU events. There was also no association between dose, 70 (RBE) versus 72.5 (RBE), and CTCAE v3.0 grade 3 or higher GU events (p = .7019).
The actuarial rate of grade 3 gastrointestinal (GI) toxicity at 5 years was 0.5% scored both prospectively according to CTCAE v3.0 and retrospectively by CTCAE v4.0 criteria (CI, 0.1%–3.3%); no grade 4 or 5 GI toxicities were observed. The rates of 5-year freedom from grade 2 + rectal bleeding and proctitis were 91.7% and 85.6% for low- and intermediate-risk patients, respectively, and 89.0% overall. The number of patients who developed CTCAE v3.0 grade 2 and 3 toxicities were 11 and 0 for low-risk patients and 12 and 1 for intermediate-risk patients, respectively. There was no association between prostate size and GI events.
Median IPSS before treatment and at 4 + years after treatment were 6 and 5 for low-risk patients and 4 and 6 for intermediate-risk patients.
Survival and disease control
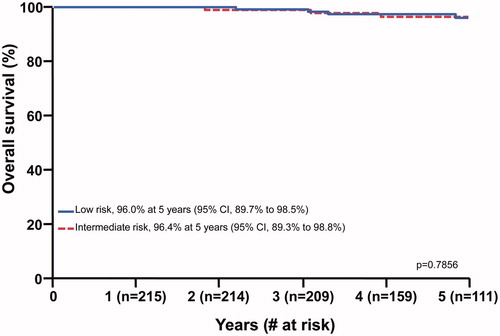
Eight patients have died of intercurrent disease (n = 6) or prostate cancer (n = 2), including 5 low-risk patients and 3 intermediate-risk patients. The 5-year overall survival rates for low-risk and intermediate-risk patients were 96.0% (CI, 89.7% to 98.5%) and 96.4% (CI, 89.3% to 98.8%), respectively (p = .7856) ().
Figure 1. Overall survival following accelerated hypofractionated proton therapy for low-risk and intermediate-risk prostate cancer at 5 years.
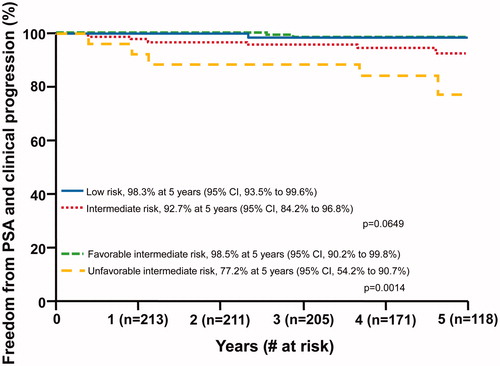
Eight patients (2 low-risk and 6 intermediate-risk) had disease progression. The 5-year freedom from biochemical and/or clinical progression rates were 98.3% (CI, 93.5% to 99.6%) for low-risk and 92.7% (CI, 84.2% to 96.8%) for intermediate-risk patients overall (p = .0649) (). Within the intermediate-risk population, 5-year freedom from biochemical and/or clinical progression rates were 98.5% for favorable intermediate-risk and 77.2% for unfavorable intermediate-risk patients, respectively (p = .0014; ). There was no association between dose, 70 (RBE) versus 72.5 (RBE), and disease recurrence (p = .7546).
Patterns of PSA response and disease progression
The median PSA nadir for the 207 patients without PSA progression was 0.3 ng/ml (range, 0–3.4); 198 patients (95.7%) had a PSA nadir <1.0 and 73.9% of nadirs were <0.5 ng/ml. The median time to nadir was 39 months for both low-risk and intermediate-risk patients. The median time to PSA and/or clinical progression was 29 months (range, 4–57 months).
Patterns of progression are shown in . PSA progression occurred in 7 patients, either with positive prostate biopsy (n = 2), isolated pelvic node failure (n = 2), or distant metastases (n = 3). An 8th patient with low-risk disease had an isolated clinically detected biopsy-proven local failure with a PSA rise that did not meet the definition of PSA failure. There were no failures in the subset of low-risk patients with very low-risk disease. Disease progression occurred in 2 (1.6%) of 120 low-risk patients, 1 (1.4%) of 69 favorable intermediate-risk patients, and 5 (19.2%) of 26 unfavorable intermediate-risk patients. The 5-year rate of freedom from distant metastases was 98.5% in low- and favorable-risk patients compared with 88.5% in unfavorable-risk patients (p < .0001; ).
Figure 3. Freedom from distant metastases for low-risk and favorable-risk patients compared with unfavorable-risk patients.
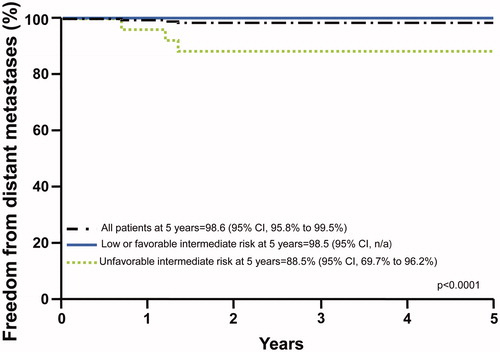
Table 2. Patterns of disease progression.
Discussion
With modern conformal photon techniques, such as intensity-modulated radiotherapy (IMRT), radiation doses for prostate cancer can be escalated to as high as 86.4 Gy in 48 fractions using 1.8 Gy per fraction (9½ weeks) with level I evidence of improved disease control and a low risk of rectal injury [Citation11–15]. However, such treatment protraction increases the cost to the patient and the healthcare system. Moderate hypofractionation (2.4 Gy–4 Gy per fraction) offers the potential benefits of patient convenience, better resource utilization, and reduced cost. Evidence is accumulating that moderate hypofractionation with photons is both effective and safe [Citation16].
Kupelian et al. [Citation4] reported late grade 3 and 4 GI toxicity rates of 1.3 and 0.1% and GU toxicity rates of 1 and 0% in 770 consecutive patients with localized prostate cancer treated with IMRT to 70 Gy in 28 fractions at 2.5 Gy per fraction (5½ weeks) at a median follow-up up of 45 months. Six prospective randomized studies comparing standard and moderately hypofractionated radiation therapy using modern conformal radiation techniques have been reported that have most commonly shown no significant difference between fractionation arms for either disease control or toxicity parameters (). One three-armed study, which included two hypofractionated treatment arms, suggested that while 60 Gy in 20 fractions appeared non-inferior to 74 Gy in 37 fractions, 57 Gy in 19 fractions might be less effective [Citation13]. Several studies showed non-significant trends to a slightly higher rate of late effects with hypofractioned regimens [Citation13,Citation17–20]. The NRG Oncology RTOG 0415 trial compared 70 Gy in 28 fractions (the same moderately hypofractionated regimen as used in our study) with 73.8 Gy in 41 fractions in 1092 low-risk prostate cancer patients [Citation20]. At a median follow-up of 5.8 years, 70 Gy in 28 fractions was non-inferior to 73.8 Gy in 41 fractions with estimated 5-year disease-free survival rates of 86.3% (95% CI, 83.1–89) and 85.3% (95% CI, 81.9–88.1), respectively; grade 3 or higher GI and GU toxicity rates for the hypofractionation arm were 4.1% and 3.5%, respectively. Our study of AHPT used the same total dose and fraction size for low-risk patients as the 0415 hypofractonation arm and observed a freedom from disease progression rate of 98.3% for low-risk prostate cancer and grade 3 + GI and GU toxicity rates of 0.5% and 1.7% at 5 years. While providing context, these results raise questions about differences in patient selection, treatment planning, targeting, and possible differences in radiobiological effectiveness (RBE) with protons [Citation21].
Table 3. Review of disease control and toxicity.
At the time our study was designed, the role of short-term androgen deprivation therapy (ADT) in patients with unfavorable intermediate-risk prostate cancer was not defined; because our early outcomes without ADT were excellent, ADT was not used on this study. Although favorable intermediate-risk prostate cancer patients continued to have an excellent disease control rate of 98.5%, those with unfavorable intermediate-risk prostate cancer (dominant Gleason of 4, PSA >15, and/or CST2C disease) had a 5-year FFBP rate of only 77%, suggesting a possible opportunity for improvement with the addition of short-term ADT.
With the caveat that enrollment criteria were more stringent on the AHPT trial (IPSS of ≤15; no use of alpha-blockers; no diabetes mellitus; no prior intrapelvic surgery; no current or continuing use of anticoagulation, and no saw palmetto or immunosuppressants), the rates of freedom from progression and toxicity have been similar to those achieved with standard fractionated proton therapy at the same institution [Citation6,Citation22,Citation23]. The 5-year rates of freedom from biochemical progression for low- and intermediate-risk patients of 98.3% and 92.7% were comparable to rates achieved on prospective clinical trials of standard fractionated proton therapy using similar treatment techniques for low- and intermediate-risk disease (99% and 99%) [Citation6]. The CTCAE v3 grade 3 GI and GU toxicities of 0.5% and 1.7% were also similar to those observed after standard fractionation (1.0% and 5.4%). The AHPT results in the current report also appear comparable to those in a recent study of 1327 patients treated with proton therapy with standard fractionation, similar treatment techniques, and a slightly longer median follow-up of 5.5 years [Citation23], which included 544 men with low-risk and 550 with intermediate-risk disease treated with standard 2 Gy(RBE) daily fractionation; 5-year rates of freedom from biochemical progression of 99% and 94%, respectively. The CTCAE v3 and v4 grade 3 + GU toxicity rates were 4.7 and 2.9%, respectively, and the grade 3 + GI toxicity rates were 0.6% for both CTCAE v3 and v4. A subset of 589 low- and intermediate-risk patients from this study of 1327 patients treated with conventionally fractionated proton therapy was identified who met the more selective inclusion criteria in the current report of AHPT (no diabetes mellitus, TURP, alpha-blockers, or anticoagulation and IPSS <15). This patient subset had 5-year grade 3 + GU toxicity rates of 2.0% (CTCAE v3) and 0.8% (CTCAE v4), and 5-year grade 3 + GI toxicity rates of 0.7% for both CTCAE v3 and v4. A comparison of these results with those of the current trial of AHPT trial demonstrates no statistically significant difference for either GU v3 (p = .80), GU v4 (p = .26), or GI v3 or v4 (p = .77), and therefore no suggestion of increased toxicity with AHPT. A successor trial with less stringent inclusion criteria has completed enrollment and will determine the safety and efficacy of moderate hypofractionation in men with larger prostates and additional co-morbidities. It is not clear whether the PTV expansion used in this study is required or whether, in this carefully staged population, stage migration could have contributed to the excellent outcomes.
Conclusions
Five-year outcomes with image-guided AHPT for low- and intermediate-risk prostate cancer include high efficacy and minimal toxicity and compare favorably to outcomes with achieved with both standard fractionation proton therapy and hypofractionated photon therapy (). Image-guided AHPT is a viable treatment approach for men with low- or intermediate-risk prostate cancer who meet the inclusion requirements used in the study (prostate volume <60 cm3, IPSS score <15, absence of diabetes mellitus, no requirement for ongoing anticoagulation or alpha-blockers, and achievement of OAR constraints).
IONC_1287946_Supplemental_material.docx
Download MS Word (12.8 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–3868.
- Arcangeli S, Strigari L, Gomellini S, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–1178.
- Arcangeli G, Fowler J, Gomellini S, et al. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;79:1013–1021.
- Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68:1424–1430.
- Kim YJ, Cho KH, Pyo HR, et al. A phase II study of hypofractionated proton therapy for prostate cancer. Acta Oncol. 2013;52:477–485.
- Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:596–602.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE); 2006 [Internet] [cited 2006 Aug 9, 2015 Sep 1]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200.
- Mendenhall NP, Li Z, Hoppe BS, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:213–221.
- Lukka H, Hayter C, Julian JA, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23:6132–6138.
- Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692.
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060.
- Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996.
- Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74.
- Lee WR. Prostate cancer and the hypofractionation hypothesis. J Clin Oncol. 2013;31:3849–3851.
- Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464–474.
- Kuban DA, Nogueras-Gonzalez GM, Hamblin L, et al. Preliminary report of a randomized dose escalation trial for prostate cancer using hypofractionation. Int J Radiat Oncol. 2010;78:S58–SS9.
- Hoffman KE, Voong KR, Pugh TJ, et al. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: results from a randomized trial. Int J Radiat Oncol Biol Phys. 2014;88:1074–1084.
- Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–2332.
- Carabe A, Espana S, Grassberger C, et al. Clinical consequences of relative biological effectiveness variations in proton radiotherapy of the prostate, brain and liver. Phys Med Biol. 2013;58:2103–2117.
- Colaco RJ, Hoppe BS, Flampouri S, et al. Rectal toxicity after proton therapy for prostate cancer: an analysis of outcomes of prospective studies conducted at the university of Florida Proton Therapy Institute. Int J Radiat Oncol Biol Phys. 2015;91:172–181.
- Bryant C, Smith TL, Henderson RH, et al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95:422–434.