Abstract
Background: Parathyroid carcinoma (PC) is rare and diagnostically challenging. Reported outcomes are rather poor and the incidence might be increasing.
Material and methods: We performed a nationwide study on all cases (n= 32) diagnosed in 2000–2011 in Finland, and compared clinical and histopathological characteristics and outcome to atypical parathyroid (APA; n= 28) and parathyroid adenomas (PA; n= 72). The incidence in years 1955–1999 was compared to that in 2000–2013.
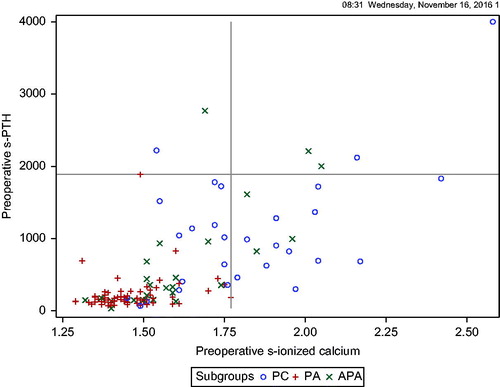
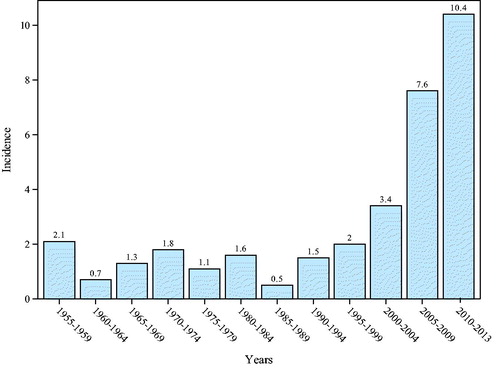
Results: Preoperatively, calcium and parathyroid hormone concentrations were higher in PC compared to APA and PA (1.76, 1.56 and 1.44 mmol/l, p < .001; and 989, 355 and 160 μmol/l, p < .001, respectively). Calcium was ≤1.77 mmol/l for all PAs. Hospitalization (44% vs. 22% and 3%, respectively, p = .01), renal (50% vs. 48% vs. 22%, respectively, p = .01) and bone (47% vs. 15% vs. 38%, respectively p = .002) manifestations were more common. PC and APA tumors were larger than PA (p < .001). Histopathological characteristics of PC compared to PA are increased mitotic activity (p= .001), chief cells (p = .003), diffuse growth pattern (p < .001), higher Ki67 (p< .001) and negative parafibromin (p < .001). One PC (1/18) and one APA (1/16) patient had a CDC73 mutation. After 6.7 (2–13.9) years of follow-up, 9.4% of PC had residual, 21% recurrent disease and 12.5% died of disease. Overall mortality did not differ between subgroups (p = .094). Recurrent PC was characterized by vascular invasion, lymph node metastases, high mitotic activity, necrosis and negative parafibromin. Incidence increased from 1.42 (range 0.52–2.14) to 7.14 (range 3.42–10.38)/10.000.000/years; (p < .001).
Conclusions: PC associates with severe primary hyperparathyroidism and must be suspected if calcium ≥1.77 mmol/l. The prevalence of CDC73 germline mutations in PC and APA in Finland is 6%. PC has distinct histopathological characteristics and its incidence has increased over the past decades.
Introduction
Parathyroid carcinoma (PC) is a rare cause of primary hyperparathyroidism (PHPT) [Citation1], found in approximately 1% of PHPT cases and the least common endocrine cancer worldwide [Citation2–5]. Recent reports indicate increasing incidence of PC in the United States in 1988 to 2003 [Citation6]), and in Australia in 2000–2010 compared to 1991–2000 [Citation7]. Diagnosis of PC is challenging. Although patients suffering from PC usually present with higher serum calcium and parathyroid hormone (PTH) concentrations compared to those with adenomas, there are no preoperative markers for PC. The diagnostic WHO criteria for PC are metastatic disease, or vascular, perineural or capsular tumor invasion [Citation8]. The lack of resection margins sometimes makes the task of the pathologist impossible [Citation5,Citation9]. PC is regarded as a rather aggressive disease, with recurrence rates of 40–60% [Citation9,Citation10], and a median overall survival of 14.3 years [Citation10]. Atypical adenomas (APA) are borderline parathyroid tumors sharing similar histological features with PC but lacking evident invasion and metastasis [Citation8]. Morphology of APA and PC include diffuse growth pattern, fibrous septa, necrosis, high mitotic activity as well as negative parafibromin stain and increased Ki67 proliferation index (PI) [Citation11–13]. Parafibromin is often absent or reduced in PC, and this protein is encoded by the CDC73 gene, which may be mutated in up to 70% of sporadic PCs [Citation14–18].
The only potential cure for PC is radical surgery with sufficient margins (en bloc resection) [Citation4,Citation10,Citation19,Citation20]. Surgery, when possible, is first-line treatment also for recurrent disease [Citation21]. Because the disease is so rare, there are no randomized, controlled trials.
Most studies on PC are register-based, lacking combined and detailed clinical and histological data [Citation22–24]. To improve the understanding of this rare disease, we performed a nationwide study on PC in Finland. We here present clinical and histopathological data and outcome of PC compared to APA and PA in Finland, and report on the incidence of PC in years 1955–1999 compared to 2000–2013.
Material and methods
Patients
Study patients
This retrospective nationwide study comprises clinical data and tissue specimen from all patients diagnosed with parathyroid cancer (PC; n = 32) in Finland during 2000–2011. These patients were identified from the Finnish Cancer Registry, the database of the Department of Pathology (Huslab and University of Helsinki, Finland) and the hospital databases of the five Finnish University Hospitals and eight Finnish Central hospitals using the ICD-10 code (C75.0). One patient had a previously identified deletion in the CDC73 gene [Citation25]. The PC group was compared to all atypical parathyroid adenoma (APA; n = 28) cases diagnosed during 2000–2011 that were found in the Helsinki pathology database. The third subgroup consisted of 72 operated parathyroid adenoma patients (PA), matched for age and gender regards the PC group, also retrieved from the Helsinki pathology database. None of the patients had MEN-1.
Re-evaluation
The diagnosis of PC was confirmed by re-evaluation of the primary tumors (J.A. and E.R.). PCs were confirmed to have either unequivocal invasion (capsular, perineural or vascular) and/or distant, regional, or lymph node metastasis, according to the 2004 WHO classification [Citation8]. Seven of 39 patients originally classified as PC were excluded from the study: three because PC could not be confirmed, and four because they were re-classified as APA.
Data collection
Histological, clinical and surgical and follow-up data including reoperations and other given treatments were collected. Laboratory data was available from the time of preoperative evaluation until end of each patient’s follow-up. Primary surgery was considered successful if, on the first postoperative day, serum PTH had decreased by 50%, and serum ionized calcium was below the upper limit of the reference range (in cases with severe hypercalcemia within one month). The operations were classified as local resection (removal of the tumor following the peritumoral capsule), or en bloc/radical resection (consisting in minimum of parathyroidectomy with removal of the ipsilateral thyroid lobe and, in some cases, adjacent structures or lymph nodes). The diagnosis of recurrent PC was based on increased serum ionized calcium and PTH concentrations and surgical findings implicating PC confirmed by histopathology. In 2016, CDC73 mutation analysis was offered to all PC and APA patients not previously screened. Altogether 56% of the PC and 57% of the APA patients were investigated.
Incidence and survival
Patients were followed until death or the last follow-up date (30 August 2015). Survival data, causes of death and the population data during the years 1955–2013 in Finland were obtained from Statistics Finland (www.statistics.fi). The nationwide number of PC cases during 1955–2000 were retrieved from the Finnish Cancer Registry.
Laboratory assays and localization studies
All laboratory tests were performed using in-house methods at the laboratories of each hospital. Serum ionized calcium (Ca-ion) was measured by ion-specific electrodes (normal range 1.15–1.30 mmol/l), and serum PTH by a chemiluminometric assays (normal range 15–65 ng/l). Serum 25-hydroxyvitamin D (25OHD, normal range >50 nmol/l) was measured by high-performance liquid chromatography (HPLC), serum creatinine (normal range 50–90 μmol/l for women; 60–100 μmol/l for men) by enzymatic photometric assay, and serum alkaline phosphatase (normal range 35–105 IU/l) and serum phosphate (normal range 0.76–1.41 mmol/l for women, 0.71–1.53 mmol/l for men) by photometric methods.
All study subjects underwent preoperative tumor localization studies including ultrasound and dual-phase scintigraphy with 99Technetium-sestamibi alone or in combination with 123Iodide. Some patients underwent additional imaging with computer tomography or magnetic resonance imaging. The imaging result was considered accurate if it represented a true positive finding, as confirmed by surgery and histopathology, without false negative or false positive findings.
Targeted next generation sequencing and multiplex ligation-dependent probe amplification analysis of the CDC73 gene
Genomic DNA was isolated from peripheral blood samples using standard methods for the detection of germline mutations. Haloplex Target Enrichment System (Agilent Technologies, Santa Clara, CA, USA) was used to capture the 17 exons of the CDC73 gene for next generation sequencing. Online design tool SureDesign (https://earray.cham.agilent.com/suredesign/) was used for capturing the probe design. Target regions consisted of exons, UTRs and 10bp flanking regions of the CDC73 gene from the RefSeq database (GRCh37/hg19). Total length of the target region was 133 kb of genomic DNA on chromosome 1q31.2. Library preparation was performed using HaloPlex Target Enrichment Kit following the manufacturer’s instructions. Paired-end sequencing (2300 bp) was performed on MiSeq instrument (Illumina, San Diego, CA, USA) using the MiSeq Reagent Kits v3 (600 cycles). Data analysis and variant calling was performed as previously described [Citation26]. An MLPA kit P466-A1 CDC73 (MRC Holland, Amsterdam, Netherlands) was used for detection of deletions and duplications of one or more exons according to the manufacturer’s instructions.
Histological studies and preparation of tissue microarrays
Formalin-fixed and paraffin-embedded tissue samples of the primary operations were retrieved for all patients and reexamined. The following histological features were evaluated (J.A. and E.R.): (1) dominant cell type, (2) growth pattern, (3) fibrous bands, (4) cystic changes, (5) hemosiderin deposits, (6) necrosis, (7) mitotic count per 10 high-power fields (HPF), (8) pathological (= atypical) mitoses, (9) nuclear atypia, (10) vascular, (11) capsular and (12) neural invasion. The cut-off for increased number of mitoses is >0 per every 10 cells counted and srong mitotic activity is >1 per every 10 cells counted.
Two patients (one PC patient and one APA patient) could not be included in the constructed tissue microarray (TMA) as there was not sufficient tissue material left. For construction of TMA blocks, we selected the histopathologically most representative areas of the tumor specimen. For PC and APA, six cores were sampled from the tumor block with 1.0 mm punchers by a semiautomatic tissue microarrayer (Beecher Instruments, Silver Spring, MD, USA; MTABooster® Version 1.01 for Beecher Manual Arrayer, Alphelys, Plaisir, France), and inserted into a recipient paraffin block. The corresponding number for benign adenomas (PA) was three cores.
Immunohistochemistry
The TMA blocks were cut into 4-μm sections, and processed through deparaffinization in xylene and rehydration with graded alcohol series. The MIB-1 slides for calculation of Ki67 indices were treated in a pretreatment (PT) module (DAKO PT Link) in Tris-HCl buffer (pH 8.5) for 20 min at 98 °C and the parafibromin slides were pretreated with Tris-EDTA in the PT module. Immunostainings were performed in an Autostainer 480 (LabVision Thermo Scientific, UK Ltd, Cheshire, UK). The MIB-1 and parafibromin slides were incubated in 0.3% Dako REAL Peroxidase-Blocking Solution (Dako, S2023) for 5 min to block endogenous peroxidase. The MIB-1 (Dako, Santa Clara, CA, USA, M7240, Clone MIB-1) primary antibody was used in a dilution 1:100 for one hour incubation and the parafibromin (Santa Cruz Technologies, Dallas, TX, USA, sc-33638, Clone 2H1) antibody immunohistochemistry was done in a dilution of 1:300 for 30 min incubation. Thereafter, followed by 30 min incubation with Dako REAL EnVision/HRP detection system, Rabbit/Mouse (ENV) reagent (Dako, K5007), the visualization of staining was done by Dako REAL DAB + Chromogen (Dako, K5007) for 10 min. Washing with PBS-0.04%-Tween20 took place between each step. PTH immunochemistry was done in fully automated instrument, Ventana Benchmark XT. As a pretreatment buffer was used CC1 for 60 min. PTH (Novocastra, Buffalo Grove, IL, USA, NCL-PTH-448, Clone 105G7) was diluted into 1:100 and incubated for 30 min. Detection kit was UltraView DAB (Ventana, Tuscon, AZ, USA, 760-500). All the three stainings were counterstained with Mayer’s hematoxylin and mounted in mounting medium. Parathyroid tissue was used as a positive control for PTH. A parathyroid carcinoma was used as negative control for parafibromin, and a parathyroid adenomas as a positive control.
Scoring of immunohistochemistry
The Ki67 proliferation index by MIB-1 staining was assessed by the Immunoratio-program as described by Tuominen et al. [Citation27]. The area with the highest MIB count was chosen for Immunoratio imaging (Magnification 400×). The results were analyzed by a pathologist (H.L.) blinded to the clinical data.
Scoring of parafibromin and PTH was done independently by two researchers (H.L. and E.R.), without knowledge of the clinical data. In case of disagreement, a consensus was done (kappa 0.90 for parafibromin, and 1.00 for PTH). The parafibromin staining was considered positive (Citation2) if >95% of the nuclei of the neoplastic cells in the TMA spot were positive, and negative (0) if >99% of all these nuclei were negative [Citation28]. Counts between these cut-offs were considered as weak positives (Citation1). Cytoplasmic staining for parafibromin was considered nonspecific and disregarded. For the PTH stain, negative was denoted 0, and positive 1.
Ethics
The study was approved by the institutional review board of Helsinki University Hospital. The study protocol was also approved by the ethics committees of the four other Finnish University Hospitals, by the National Supervisory Authority for Welfare and Health in Finland, and by the National Institute for Health and Welfare in Finland.
Statistical analysis
Statistical analysis was performed with SAS for Windows, Version 9.3 (SAS Institute, Cary, NC, USA). Absolute numbers and percentages were used to describe categorical data, age and follow-up time were presented in median and range. All other continuous data did not follow normal distribution, and were presented in medians and IQR. In the tables, number of patients is given if the information is lacking for more than 5% of the group. We assessed statistical significance between the three subgroups with Fisher’s exact test for categorical variables and with Kruskal–Wallis test for continuous variables. Correlation between immunohistochemical results of the original samples and those of TMA were evaluated by calculating Pearson’s correlation coefficient. Survival analysis was calculated with the Kaplan–Meier method. The change in the incidence of PC was assessed with chi square-test, where number of new PC cases were compared to the respective average adult population in Finland over the specific time periods (1955–2013). All incidence rates are reported as average annual incidences per 10,000,000 population, calculated over 5-year-period (except years 2010–2013 over a 4-year period). The level of significance was chosen as p value less than .05 (two-tailed).
Results
Characteristics of the study cohort
Characteristics of the study cohort are given in . There were 32 patients with PC, with 18 (56%) females and 14 (44%) males. Median age at diagnosis was 61 (17–83), 59 (31–84) and 62 (15–83) years for the PC, APA and PA subgroups, respectively (). The median age of women and men in the PC group was 68 (48–84) and 54 (17–76) years, respectively. The median follow-up time (FU) of the PC group was 6.7 (range 2.0–13.9) years. Of the PC patients, 12 (37.5%) had an additional benign parathyroid adenoma or atypical adenoma in their medical history or during follow-up, compared to one in the APA group (p= .002).
Table 1. Clinical and laboratory characteristics of the study patients (n = 132).
Biochemical characteristics and disease manifestations
Biochemical characteristics and disease manifestations are presented in . There were no nonfunctioning tumors. At the time of diagnosis, Ca-ion, PTH and creatinine concentrations differed significantly between PC, APA and PA subgroups (). The highest Ca-ion and PTH concentration in the APA and the PA groups were 2.05 mmol/l and 2769 ng/l, and 1.77 mmol/l and 1887 ng/l, respectively. The lowest corresponding values in PC were 1.38 mmol/l and 68 ng/l (). Preoperative hospitalization because of marked hypercalcemia or hypercalcemic crisis (Ca-ion >1.75 mmol/l) was needed for 44, 22 and 3% of PC, APA and PA subgroups, respectively (p < .001). The prevalence of renal (renal failure, kidney stones) and bone (pathological fractures, osteoporosis, Brown tumors) manifestations differed significantly between the subgroups (p = .010 and p = .002, respectively) ().
Preoperative localization studies
Preoperative ultrasound imaging was accurate in 77, 43 and 39% of PC (n = 26), APA (n = 23) and PA (n = 64) subgroups, respectively (p< .001). Planar isotope imaging was accurate in 77, 64 and 70% of PC (n = 26), APA (n = 25) and PA (n = 68) subgroups, respectively (p = .62).
Surgery
Of the PC, APA and PA subgroups, 91, 93 and 91% were cured by primary surgery; the postoperative status of one APA patient remains unknown. Of the PC, APA and PA subgroups, 53, 82 and 92%, respectively (p < .001), underwent local resection. The corresponding figures for en bloc or radical resection were 47, 18 and 8%, respectively (p < .001). The corresponding numbers for tumor weight and size were 4.8 (2.8–14.0) g, 2.6 (1.3–4.5) g and 0.76 (0.43–1.5) g, (p < .001); and 2.95 (2.0–3.0) cm, 2.0 (1.4–3.0) cm and 1.6 (1.2–2.0) cm (p < .001), respectively. At primary surgery, additional pathological parathyroid glands (n = 8) were identified in PC (n = 5) only. The histopathological diagnoses were as follows: PC (n = 1, on the contralateral side of the neck), APA (n = 1), PA (n = 5) and hyperplasia (n = 1). In PC, tumor invasion to the thyroid gland, to the surrounding muscle tissue and to the trachea was detected in one, one, and one patient, respectively. Two patients had lymph node metastases.
Histological findings
Histological findings are presented in and in .
Figure 2. Diagnostic (A–B) and typical (C–F) findings in parathyroid carcinoma. A: Infiltrative growth to adipose tissue, B: Vascular invasion, C: Diffuse growth pattern, and 100% chief cells, D: Nuclear atypia, E: Fibrous bands and hemosiderin, F: Necrosis.
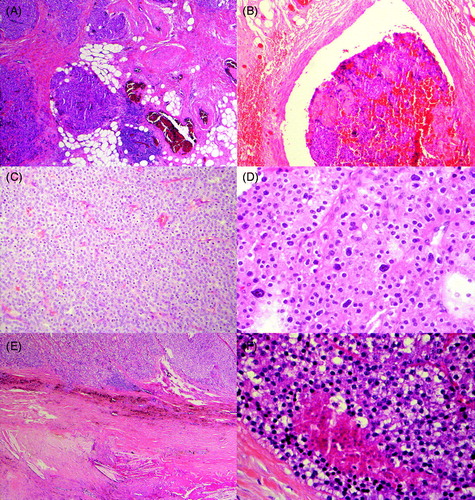
Table 2. Histopathological findings in the different subgroups.
Invasion
Capsular, vascular and perineural invasion was present in 72, 72 and 9% of the PC specimens. By definition, there was no invasion in the APA and PA subgroups.
Mitotic activity
Increased mitotic activity (>0 cells per 10 cells counted) characterized PC compared to APA and PA (25, 9 and 1%, respectively, p < .001). Mitotic activity was particularly strong (observed in >1/10 cells) in 2 of 32 PC and 1 of 28 APA samples.
Cell type
Chief cells (100%) was the dominant cell type in PC and APA compared to PA (69, 68 and 41%, respectively, p < .001), while oncocytic cells were more common in APA and PA compared to PC (16, 20 and 9%, respectively, p = .079, ).
Growth pattern
There were significant differences in the growth patterns between the subgroups. Diffuse growth pattern was more common in PC compared to APA and PA (overall distribution; p < .001, ), while follicular growth pattern was more common in A and APA compared to PC (overall distribution; p < .007), with no clear differences in cystic or islet-cell growth patterns between the subgroups ().
Necrosis, hemosiderin deposits, fibrous bands and cysts
There were significant differences in the distribution patterns of all the aforementioned parameters (). Necrosis was present in PC (3 of 32) only (p < .05). Hemosiderin deposits and fibrous septae were more common in PC and APA compared to PA (56, 61 and 1%, p < .001; and 88, 93 and 3%, p < .001), respectively (). Cysts were most common in PA (53%) compared to APA (44%) and PC (31%) (overall distribution, p < .05).
Immunohistochemistry
Immunohistochemical data are presented in and . PTH stain was positive in all samples (100%). In PC, APA and PA subgroups, parafibromin stain was positive (>95% of cell nuclei stained) in 22, 33 and 76% of cases, respectively (p < .001) (). The corresponding percentages for negative parafibromin stain were 13, 4 and 0%, respectively.
Figure 3. Parafibromin stain in parathyroid carcinomas (A negative and B positive). Digital images of Ki-67 staining in Tissue Micro Array in a parathyroid adenoma with a low PI (C) and in an aggressive parathyroid carcinoma with high PI (D). The corresponding pseudocolor image of Immunoratio-program respectively with low PI (E) and high PI (F).
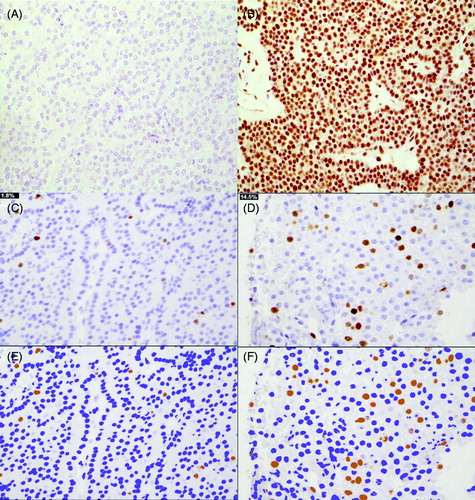
Table 3. Parafibromin and Ki-67 proliferation indices and germ-line CDC73 mutation status in the different subgroups.
Figure 4. Survival analysis in parathyroid carcinoma, atypical adenoma and parathyroid adenoma. 5-year and 10-year survivals are 90.6 and 71.9% for PC, 83.9 and 73.4% for APA, and 91.7 and 82.5% for PA (p = NS).
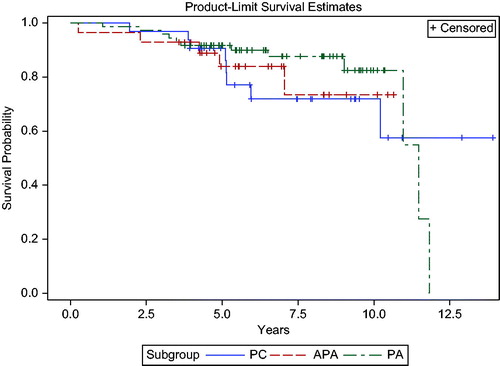
Table 4. Recurrences, treatments and survival in the different subgroups.
Table 5. Clinical, biochemical and histological findings in patients with recurrent vs cured parathyroid carcinoma (PC).
Ki-67 indices analyzed by Immunoratio (TMA) were significantly higher in PC and APA compared to PA (2.7% vs. 2.0% vs. 0.9%, p < .001) (). Ki-67 percentages of the original samples (PC 5.0%, APA 2.0%), correlated with those of the TMA (r= 0.42, p = .03; and r= 0.72, p < .001, respectively).
CDC73 mutation analysis
The CDC73 germline mutation status for PC and APA, in relation to parafibromin stain, are given in . One 18-year-old man with PC, with negative parafibromin stain, had a deletion of exons 1 to 10 (25). One 31-year-old man with APA, with recurrent disease, kidney stones and weak positive parafibromin stain had a p.Arg92 Term mutation in codon 91.
Recurrences and cancer deaths
In PC, 6/29 patients (21%) cured by primary surgery recurred after a median of 24 months (range 4–60), with Ca-ion and PTH concentrations of 1.41 mmol/l (range 1.20–1.60) and 152 ng/l (range 101–1241), respectively (). One patient with chronic hypocalcemia due to severe chronic renal failure was normocalcemic (1.20 mmol/l) at recurrence. In three PC patients, PHPT recurred as an adenoma. In APA, one patient had persistent disease due to tumor rupture. In another APA patient, re-operation for persistent disease revealed PC. A third APA patient, cured by primary surgery, recurred in 23 months because of another APA, located in the same site as the primary tumor. In the PA group, one patient recurred, due to an adenoma.
Overall 5- and 10-year survivals in PC were 91 and 72%, respectively, and did not differ significantly from the other subgroups (). In PC, 4 of 32 patients (12.5%) died of disease at a median FU of 43.9 (range 23.4–61.3) months (). Four other deaths in the PC group were due to severe asthma, stroke, Merkel cell carcinoma and salivary gland carcinoma, respectively. In APA, all deaths occurred in cured patients and were unrelated to PHPT.
Treatments and treatment complications in parathyroid carcinoma
PC patients underwent on average 1.7 (range 1–5) operations for the disease (). Sixty-nine percent received calcium decreasing agents (zoledronic acid or cinacalcet), either for severe preoperative hypercalcemia or for PC not cured by surgery (p < .001 compared to APA and PA). Of the PC patients, 22 and 13% were treated with external neck radiation therapy and/or chemotherapy (temozolomide), respectively (). Seven patients () underwent radiation therapy, four after primary surgery and three after re-operation because of recurrence. Of the four patients who underwent radiation therapy after primary surgery, three died within four years, and one has not recurred during 11 years of follow-up.
Treatment complications caused by all operations during follow-up are shown in . The prevalence of transient or persistent hypocalcemia in PC, APA and PA were 56, 11 and 4%, respectively (p < .001), and for transient or persistent laryngeal nerve palsy 34, 11 and 3%, respectively (p < .001). In PC patients, preoperatively increased creatinine concentrations did not improve after surgery (p = .39) ().
Factors associated with recurrence or cancer death
Characteristics of PC patients with and without recurrence are presented in . Distant metastases were found in 5 out of 6 patients with recurrent PC, and four of them died of disease (). Median preoperative Ca-ion concentrations were slightly higher in recurrent compared to cured PC, but the difference was not statistically significant (). Median preoperative PTH concentrations did not differ significantly, but the lowest PTH concentration at diagnosis in recurrent PC was 358 ng/l, compared to 68 ng/l in the cured PC group (). Median tumor size was similar (). Recurrent PC was more often characterized by lymph node metastases compared to the cured PC group (p = .03, ). Parafibromin expression was weaker in recurrent compared to cured PC. All of the six PC tumors that recurred were characterized by vascular invasion at primary diagnosis, and all tumors characterized by necrosis (n = 3) recurred (). Recurrent PC had significantly higher mitotic activity, number of pathological mitoses, and more marked nuclear atypia (p < .05, p < .03 and p < .03, respectively; ).
Incidence of parathyroid carcinoma
illustrates the increasing incidence of PC. In the years 1955 to 2000, the mean incidence was 1.42 cases/10,000,000/year (range 0.52–2.14). During 2000 to 2013, the mean incidence was 7.14 (range 3.42–10.38) rising from 3.4 (2000–2004) to 10.4 (2010–2013), p < .001 ().
Discussion
To the best of our knowledge, this is the first study presenting a nationwide cohort on PC. As the series does not include only the most aggressive cases, it can be regarded as unbiased. The present study is one of the largest studies on PC, including 32 cases and with detailed clinical and histological comparison to the two other parathyroid tumor subgroups, APA and PA. All tumor samples were reexamined, which resulted in re-classification of 7 of originally 39 PC cases.
We demonstrate a significant increase in the incidence of PC in Finland during the last 20 years compared to the time period 1955–2000. Previous studies from the United States [Citation6] and from Australia, reported a similar increase in disease rate in years 2000–2010 compared to the previous decade [Citation7]. The increasing incidence of PC may partly be explained by improvements in the diagnostic accuracy, but may also reflect a true rise.
In this study, median age at diagnosis of PC was 60.8 years. Close to half (44%) of these patients were male in contrast to the distribution in benign PHPT (2–3 times more common in women), in line with previous data [Citation9,Citation22,Citation29]. The reason for this difference in sex distribution is unclear. At the time of diagnosis, Ca-ion, PTH and creatinine concentrations differed significantly between PC, APA and PA subgroups, with the highest concentrations observed in PC (all p < .001). In PA, PTH and Ca-ion concentrations did not exceed 1887 ng/l and 1.77 mmol/l, respectively (). According to our study, hospitalization for severe hypercalcemia as well as kidney and bone manifestations are more common in PC compared to APA and PA (all p < .001). In line with previous studies [Citation10,Citation12], postoperative hypocalcemia was significantly more common in PC compared to both APA and PA (p < .001). Of note, the preoperative mean creatinine concentration was increased in the PC group and did not return to normal after surgery.
There is little previous data on preoperative localization in PC [Citation21]. In the present study, ultrasound more accurately identified PC tumors compared to APA and PA. PCs were also larger and heavier (p < .001), as previously described [Citation12]. PC was characterized by multiglandular disease, as 35% had an additional, benign parathyroid tumor, in line with previous reports [Citation10].
Diffuse growth pattern characterized by chief cells is typical for PC, but may also be seen in other parathyroid tumor subgroups. We observed that higher mitotic activity and nuclear atypia were more common in PC, as previously reported [Citation12]. Global loss of parafibromin predicts poor outcome in PC [Citation30], but negative parafibromin alone cannot distinguish PC from APA [Citation12,Citation31]. In the present study, significant differences in parafibromin stain between the subgroups were observed (p < .001). Although parafibromin stain was weaker in PC, a completely negative stain was observed in only 13% of PC, compared to 4% of APA and 0% of PA, respectively. The proportion of PAs demonstrating relatively weak positive parafibromin stain in the present study was 24%, which might be related to differences in the staining process compared to other studies [Citation17].
None of the PCs that did not recur stained negative for parafibromin. One-third of the recurrent PCs were completely negative for parafibromin and none clearly positive. The patient with APA and a negative parafibromin stain had persistent disease and underwent reoperations.
Germ-line CDC73 mutation analysis was available for 56 and 57% of the PC and APA subgroups, respectively. One young man with early-onset PC and familial disease had a CDC73 mutation [Citation25], with a total mutation rate of 6% in this national Finnish PC cohort. In addition, a 31-year-old man with APA, recurrent disease and kidney stones had a p.Arg92 Term mutation in codon 91. The percentage of CDC73 mutations in the APA group was also 6%. Others have reported that 15% to 70% of sporadic PC cases may demonstrate somatic or germline CDC73 mutations [Citation20]. A limitation of the present study is that germ-line CDC73 mutation analysis was not available for all PC and APA patients and somatic CDC73 mutation screening was not performed. However, Cetani et al. [Citation16] demonstrated that, compared to mutation analysis, negative parafibromin stain better predicts poor outcome in PC, and that the added value of CDC73 mutation analysis is identification of germline mutations, that prompts screening of family members. Negative parafibromin stain seems to predict aggressive behavior both in APA and in PC [Citation32,Citation33].
A proliferation index >5% in a parathyroid tumor is suggestive of parathyroid carcinoma [Citation34–39]. In our study, median Ki67 differed significantly between the subgroups with higher percentages in PC, but there was considerable overlapping. All benign adenomas in the present study had a Ki67 < 5%. A limitation of TMA analysis is that all possible hot spots cannot be analyzed, as the method analyses three to six spots of each tissue sample. Consequently, the proliferation index in TMA is lower although it correlates well with that originally obtained. In contrast to the present and previous smaller series [Citation31,Citation37,Citation40], Quinn et al. [Citation12] reported that neither Ki67 nor parafibromin stain differed between PC and APA. However, as original pathology reports were used in that study, without reexamining the samples, the results may have been hampered by interobserver variability.
In the present study, half (53%) of the surgical procedures for PC were local resections, a figure close to that of the NCDB reports [Citation24]. Three patients with PC had incomplete tumor removal. During a median follow-up of 6.7 years, PC recurred in 21% of the patients, in line with the report of 23% recurrence after 55 months of follow-up by Villar del Moral et al. [Citation29]. Others report of persistent or recurrent disease in over 50% PC patients [Citation10,Citation21,Citation41]. Talat et al. [Citation9] reported a recurrence rate of 63% during 6.1 years, after exclusion of subjects characterized by capsular invasion only. Mean time of recurrence in previous reports range from 2.5 to 8.4 years. However, late recurrences (23 years after primary surgery) have been reported [Citation10,Citation21,Citation23]. In the present study, time to recurrence was 2 years. It is possible that there will be later recurrences of the present cohort after longer follow-up.
Five of the 32 PC patients developed metastatic disease and, despite all treatments, 4 (80%) of them died of disease during the 6.7-year FU. The overall 5-year-survival rate, 91%, is in line with others [Citation29], but seems slightly better than previously reported 5-year overall survivals of 85%, derived from various cancer databases and longitudinal retrospective single institution reports [Citation6,Citation10,Citation23,Citation41,Citation42]. These report 10-year overall survivals between 49 and 77% [Citation6,Citation10,Citation23,Citation41,Citation42]. In this study, the disease-related and overall mortalities of PC patients did not differ from those of APA or PA groups. Advances in diagnostics and operative techniques may in part explain the lower recurrence and mortality rates reported in these more recent studies.
Characteristics of patients with recurrent PC (n = 6) compared to cured PC were lymph node metastases at diagnosis, development of distant metastases during FU, weaker parafibromin stain and vascular invasion. Some [Citation10,Citation29] but not all previous studies [Citation6,Citation23] reported an association between recurrent PC and lymph node metastases. Vascular invasion seems to predict aggressive behavior [Citation9,Citation43]. In addition, in the present study, all tumors characterized by necrosis recurred. As observed previously [Citation29], recurrent PCs were characterized by higher mitotic activity and pathological mitoses, and more marked nuclear atypia (p < .05, p < .03 and p < .03, respectively). Parafibromin stain cannot be used as a diagnostic marker of PC but a negative stain warrants closer follow-up.
Conclusions
PC is characterized by a significantly worse clinical course compared to APA and PA, including bone and renal manifestations and hospitalization due to a severe hypercalcemia. PC should be suspected preoperatively in all PHPT patients characterized by Ca-ion concentrations ≥1.77 mmol/l. However, the lowest observed preoperative Ca-ion in PC was 1.38 mmol/l. Histopathological characteristics of recurrent PC include lymph node metastasis, vascular invasion, necrosis, negative parafibromin stain and Ki67 > 5%. The prevalence of CDC73 germline mutations in Finnish patients with PC and APA is approximately 6%. The incidence of PC has clearly increased in Finland during the past decades.
Acknowledgments
We thank Elina Aspiala, Eija Heiliö, Päivi Koskinen, Päivi Peltokangas, Joose Raivo, Satu Remes and Samppa Ryhänen for their excellent technical assistance.
Disclosure statement
The authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Funding
References
- De Quervain F. Parastruma maligna aberata. 1904;100:334–352.
- Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol. 2012;13:11–23.
- Sharretts JM, Kebebew E, Simonds WF. Parathyroid cancer. Semin Oncol. 2010;37:580–590.
- Schulte KM, Talat N. Diagnosis and management of parathyroid cancer. Nat Rev Endocrinol. 2012;8:612–622.
- Marcocci C, Cetani F, Rubin MR, et al. Parathyroid carcinoma. J Bone Miner Res. 2008;23:1869–1880.
- Lee PK, Jarosek SL, Virnig BA, et al. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736–1741.
- Brown S, O'neill C, Suliburk J, et al. Parathyroid carcinoma: increasing incidence and changing presentation. ANZ J Surg. 2011;81:528–532.
- Bondeson L, Grimelius L, DeLellis RA, et al. Parathyroid carcinoma and Parathyroid adenoma. In: DeLellis RA, Lloyd RV, Heitz P, et al., editors. Pathology and genetics. tumors of endocrine organs. WHO Classification of Tumours. Lyon: IARC Press; 2004. p. 124–131.
- Talat N, Schulte KM. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann Surg Oncol. 2010;17:2156–2174.
- Harari A, Waring A, Fernandez-Ranvier G, et al. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab. 2011;96:3679–3686.
- Tan MH, Morrison C, Wang P, et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin Cancer Res. 2004;10:6629–6637.
- Quinn CE, Healy J, Lebastchi AH, et al. Modern experience with aggressive parathyroid tumors in a high-volume New England referral center. J Am Coll Surg. 2015;220:1054–1062.
- Ippolito G, Palazzo FF, Sebag F, et al. Intraoperative diagnosis and treatment of parathyroid cancer and atypical parathyroid adenoma. Br J Surg. 2007;94:566–570.
- Howell VM, Haven CJ, Kahnoski K, et al. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet. 2003;40:657–663.
- Cetani F, Ambrogini E, Viacava P, et al. Should parafibromin staining replace HRTP2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma? Eur J Endocrinol. 2007;156:547–554.
- Cetani F, Banti C, Pardi E, et al. CDC73 mutational status and loss of parafibromin in the outcome of parathyroid cancer. Endocr Connect. 2013;2:186–195.
- Juhlin CC, Villablanca A, Sandelin K, et al. Parafibromin immunoreactivity: its use as an additional diagnostic marker for parathyroid tumor classification. Endocr Relat Cancer. 2007;14:501–512.
- Shattuck TM, Valimaki S, Obara T, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–1729.
- Schulte KM, Talat N, Galata G, et al. Oncologic resection achieving r0 margins improves disease-free survival in parathyroid cancer. Ann Surg Oncol. 2014;21:1891–1897.
- Cetani F, Pardi E, Marcocci C. Update on parathyroid carcinoma. J Endocrinol Invest. 2016;39:595–606.
- Kebebew E, Arici C, Duh QY, et al. Localization and reoperation results for persistent and recurrent parathyroid carcinoma. Arch Surg. 2001;136:878–885.
- Sadler C, Gow KW, Beierle EA, et al. Parathyroid carcinoma in more than 1000 patients: a population-level analysis. Surgery. 2014;156:1622–1629; discussion 1629-–630.
- Hundahl SA, Fleming ID, Fremgen AM, et al. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: a National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538–544.
- Asare EA, Sturgeon C, Winchester DJ, et al. Parathyroid carcinoma: an update on treatment outcomes and prognostic factors from the National Cancer Data Base (NCDB). Ann Surg Oncol. 2015;22:3990–3995.
- Korpi-Hyövälti E, Cranston T, Ryhänen E, et al. CDC73 intragenic deletion in familial primary hyperparathyroidism associated with parathyroid carcinoma. J Clin Endocrinol Metab. 2014;99:3044–3048.
- Huopio H, Miettinen PJ, Ilonen J, et al. Clinical, genetic, and biochemical characteristics of early-onset diabetes in the Finnish population. J Clin Endocrinol Metab. 2016;101:3018–3026.
- Tuominen VJ, Ruotoistenmäki S, Viitanen A, et al. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010;12:R56.
- Kim HK, Oh YL, Kim SH, et al. Parafibromin immunohistochemical staining to differentiate parathyroid carcinoma from parathyroid adenoma. Head Neck. 2012;34:201–206.
- Villar-del-Moral J, Jimenez-Garcia A, Salvador-Egea P, et al. Prognostic factors and staging systems in parathyroid cancer: a multicenter cohort study. Surgery. 2014;156:1132–1144.
- Witteveen JE, Hamdy NA, Dekkers OM, et al. Downregulation of CASR expression and global loss of parafibromin staining are strong negative determinants of prognosis in parathyroid carcinoma. Mod Pathol. 2011;24:688–697.
- Truran PP, Johnson SJ, Bliss RD, et al. Parafibromin, galectin-3, PGP9.5, Ki67, and cyclin D1: using an immunohistochemical panel to aid in the diagnosis of parathyroid cancer. World J Surg. 2014;38:2845–2854.
- Howell VM, Gill A, Clarkson A, et al. Accuracy of combined protein gene product 9.5 and parafibromin markers for immunohistochemical diagnosis of parathyroid carcinoma. J Clin Endocrinol Metab. 2009;94:434–441.
- Kruijff S, Sidhu SB, Sywak MS, et al. Negative parafibromin staining predicts malignant behavior in atypical parathyroid adenomas. Ann Surg Oncol. 2014;21:426–433.
- Abbona GC, Papotti M, Gasparri G, et al. Proliferative activity in parathyroid tumors as detected by Ki-67 immunostaining. Hum Pathol. 1995;26:135–138.
- Arvai K, Nagy K, Barti-Juhasz H, et al. Molecular profiling of parathyroid hyperplasia, adenoma and carcinoma. Pathol Oncol Res. 2012;18:607–614.
- Fernandez-Ranvier GG, Khanafshar E, Tacha D, et al. Defining a molecular phenotype for benign and malignant parathyroid tumors. Cancer. 2009;115:334–344.
- Farnebo F, Auer G, Farnebo LO, et al. Evaluation of retinoblastoma and Ki-67 immunostaining as diagnostic markers of benign and malignant parathyroid disease. World J Surg. 1999;23:68–74.
- Lloyd RV, Carney JA, Ferreiro JA, et al. Immunohistochemical analysis of the cell cycle-associated antigens Ki-67 and retinoblastoma protein in parathyroid carcinomas and adenomas. Endocr Pathol. 1995;6:279–287.
- Wang O, Wang C, Nie M, et al. Novel HRPT2/CDC73 gene mutations and loss of expression of parafibromin in Chinese patients with clinically sporadic parathyroid carcinomas. PLoS One. 2012;7:e45567.
- Fernandez-Ranvier GG, Khanafshar E, Jensen K, et al. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis. Cancer. 2007;110:255–264.
- Busaidy NL, Jimenez C, Habra MA, et al. Parathyroid carcinoma: a 22-year experience. Head Neck. 2004;26:716–726.
- Sandelin K, Auer G, Bondeson L, et al. Prognostic factors in parathyroid cancer: a review of 95 cases. World J Surg. 1992;16:724–731.
- Schulte KM, Gill AJ, Barczynski M, et al. Classification of parathyroid cancer. Ann Surg Oncol. 2012;19:2620–2628.