Abstract
Background: Patients with cancer are often treated by many healthcare providers, receive complex and potentially toxic treatments that can increase the risk for iatrogenic harm. The aim of this study is to investigate whether hospitalised cancer patients are at higher risk of adverse events (AEs) compared to a general hospital population.
Material and methods: A total of 6720 patient records were retrospectively reviewed comparing AEs in hospitalised cancer patients to a general hospital population in Norway, using the IHI Global Trigger Tool method.
Results: 24.2 percent of admissions for cancer patients had an AE compared to 17.4% of admissions of other patients (p < .001, rr 1.39, 95% CI 1.19–1.62). However, cancer patients did not have a higher rate of AEs per 1000 patient days compared to other patients, 37.1 vs. 36.0 (p = .65, rr 0.94, 95% CI 0.90–1.18). No particular cancer category is at higher risk. The rate of AEs increases by 1.05 times for each day spent in hospital. For every year increase in age, the risk for AEs increases by 1.3%. Cancer patients more often have hospital-acquired infections, other surgical complications and AEs related to medications.
Conclusions: Because of higher age, longer length of stay and surgical treatment, hospitalised cancer patients experience AEs more often than other patients.
Introduction
The health care system is a complex environment involving both system and individual risk factors for iatrogenic harm. Based on patient characteristics, complexity and seriousness of the illness, some patients are at greater risk of adverse events (AEs) [Citation1,Citation2]. The risk of iatrogenic harm increases with age, length of stay, surgery, emergency services and treatment in intensive care [Citation3]. Patients with cancer are often treated by a variety of healthcare providers, receive complex and potentially toxic treatments that could increase the risk of iatrogenic harm [Citation4]. As new treatments are developed, new safety hazards will evolve. In addition, cancer patients may be more prone to AEs due to the disease itself. Accurate and reliable measurement of AEs remains a challenge in the patient safety field [Citation5]. The Institute for Healthcare Improvement’s Global Trigger Tool (IHI GTT) is widely used as a method to measure and monitor AEs in general hospitalised patients [Citation3,Citation6]. Despite this method’s high sensitivity and specificity in detecting iatrogenic harm, there are limitations [Citation7–9]. One Danish study raises methodological concerns of the IHI GTT, not being specific enough in monitoring harm in cancer patients [Citation10]. Knowledge of patient safety measures in cancer is limited, and a disease-specific approach could be of value for targeted improvements in cancer care. The aim of this study is to investigate whether cancer patients have a higher risk of AEs compared to a general hospital population as documented by the IHI Global Trigger Tool.
Material and methods
Study design
The study is a retrospective record review comparing AEs in hospitalised cancer patients and patients with other diseases.
Setting
The study was performed at a public health trust in Norway. Nordland Hospital Trust has three somatic hospitals: one central and two smaller district hospitals, with a total of 524 beds. Cancer patients are treated and hospitalised in all three hospitals, but only the central hospital has a separate department of oncology. The oncology department provides ambulatory chemotherapy, palliative care and radiotherapy. Cancer surgery is primarily performed at the Central Hospital in Bodø. None of the hospitals has a separate oncological inpatient unit, so cancer patients are admitted to other department depending on the origin of their cancer.
Study population
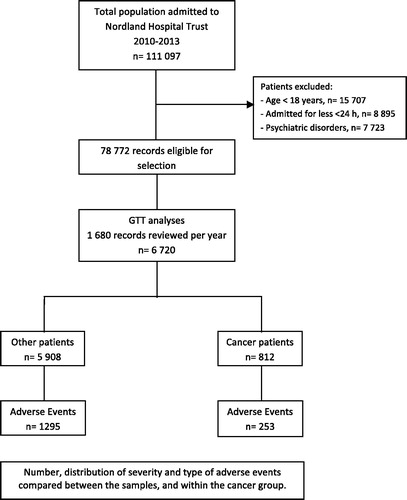
Since 2010, all hospitals in Norway are required to review a minimum of 20 randomly selected medical records per month using the IHI GTT method [Citation11]. Nordland Hospital Trust chose from the start to review 140 records monthly to achieve more accurate measurement and better support for local improvement initiatives [Citation12]. From 1 January 2010 to 31 December 2013, a total of 6720 records were reviewed using the IHI GTT method. Ten patient records were randomly sampled, block randomised twice monthly from the discharge list of seven units in the trust (surgery, orthopaedics, internal medicine, gynaecology/obstetrics, neurology/others and the district hospitals of Lofoten and Vesterålen). Patients below the age of eighteen, patients with a length of stay less than twenty-four hours or patients admitted primarily for psychiatric conditions or rehabilitation were excluded [Citation12–14]. Our analysed sample accounts for 8.5% of the eligible discharges of inpatients from the health trust in the study period. Cancer patients were identified by matching the patient ID number in the sample to cancer diagnosis in the discharge lists of the hospitals. From the total sample size of 6720 records, 812 (12.1%) of the patients had cancer as primary or secondary diagnosis on discharge classified by ICD-10. Age, gender, length of stay, type of admission, hospital, department and cancer characteristics were obtained ().
Review method
The review was done according to the Norwegian version of the IHI GTT manual. The Norwegian version is identical to the IHI GTT, except for minor changes to three triggers [Citation13,Citation14]. All review teams were trained according to the IHI protocol for GTT analyses. Seven different teams reviewed records from their unit in the trust. All review teams consist of one physician and two nurses, and only had minor changes in composition during the study period. The review was performed as in two-stages. Two nurses reviewed all records independently and then together reached consensus on presence, category and severity of AEs. The physician then authenticated their findings. Cancer patients were reviewed together with the other patients, and separated afterwards for the study. AEs were defined as ‘Unintended physical injury resulting from or contributed to by medical care that requires additional monitoring, treatment or hospitalization, or that results in death.’ [Citation14] The severity of AEs was categorised according to the NCC MERP index [Citation15] (). AEs were before reviewing grouped into 23 categories according to recommendations in the Norwegian GTT manual. For statistical purpose, the categories were aggregated into eight main categories in the study: hospital-acquired infections, surgical complications, bleeding/thrombosis, patient fall/fracture, medication harm, obstetric harm, pressure ulcer and others.
Table 1. Severity grading of AEs.
Statistical analysis
Demographic variables were compared using the Pearson’s Chi-squared test and the Mann–Whitney U-test. Incidence rates of AEs, severities and categories were compared using negative binominal regression in generalised linear models. Rates were calculated as AEs per 1000 patient days and as percentage of admissions with one or more AEs. Log patient days were used as offset variable to compare rates per 1000 patient days. For admissions with AEs, the offset variable was set to a fixed value of zero. In addition, we adjusted for demographical variables: age, gender, length of stay, type of admission, hospital, department and year of discharge. A p-value < .05 was deemed statistically significant. Data were analysed with IBM SPSS V23.0.
Results
Demographic characteristics
According to the discharge index, cancer diagnosis accounts for 10.8% of patients admitted to the total hospitals population. In our sample, cancer patients represent a stable rate of 12% per year, evenly distributed between the hospitals. Cancer patients are 10.2 years older, stay 2.27 days longer in hospital and are more often male than the general hospital population. Cancer patients are more often admitted electively, and are more likely to be admitted to a surgical department than other patients ().
Table 2. Characteristics in 6720 patients.
Cancer of the large bowel (15%), prostate (13%) and lung (12%) are most common. Gastrointestinal and urinary cancer counts for 51% of the cancers. 59% of the cancer patients have metastases and 65% are in a palliative setting. A majority of the patients (51%) received ordinary medical treatment. Thirty-five percent had surgery or other minor procedures such as biopsies, stent insertion or pleural draining. Fifteen percent received cancer-related treatments such as chemotherapy or radiation ().
Table 3. Cancer characteristics and rate of AEs.
Comparison of AEs
An AE was recorded in 24.2% of admissions for cancer patients compared to 17.4% of admissions for other patients (p < .001, rr 1.39, 95% CI 1.19–1.62). Estimating the rate per 1000 patient days, cancer patients have no higher rate of AEs than other patients, 37.1 vs. 36.0 (p = .65, rr 0.94, 95% CI 0.90–1.18). Adjusted for demographic variables, there is still no significant difference between the groups but the incidence rate of AEs decreases, 24.4 vs. 26.0 (p = .35, rr 0.94, 95% CI 0.82–1.07) ().
Table 4. Incidence rates for AEs.
For the total sample, the rate of AEs is 1.05 times greater for each extra day spent in hospital (p < .001, 95% CI 1.04–1.06). For every year increase in age, the risk of an AE increases by 1.3%, (p < .001, rr 1.013, 95% CI 1.01–1.02). Acute admission increases the risk of AEs by 17% (p = .01, rr 1.17, 95% CI 1.039–1.327). Admission to a surgical or gynaecology department increases the rate of AEs by more than 50%. The district hospital in Lofoten has a 30% lower rate of AEs (p < .001, rr 0.70, 95% CI 0.580–0.850) ().
Table 5. Incidence rate for AEs.
Cancer patients having surgery or minor procedures have an increased rate of 68% for AEs compared to other patients with cancer (p = .007, rr 1.68, 95% CI of 1.15–2.46). Receiving treatment with curative intent increases the rate of AEs by 74% (p = .002, rr 1.74, CI 95% 1.24–-2.46). However, rates are similar for the different cancer categories ().
Severity of AEs
Most of the AEs are of temporary harm, severity E and F for both cancer patients (88%) and others (89%). Adjusted for demographic variables, there is no difference in severity per admission or per 1000 patient days between cancer patients and other patients.
Type of AEs
Cancer patients more often than other patients experience hospital-acquired infections, 11.5 vs. 7.6% per admission (p = .001, rr 1.51, 95% CI 1.20–1.91). Cancer patients primarily have lower respiratory infections (4.5 vs. 2.8%) and other infections (3.2 vs. 1.6%). Cancer patients have a 54% greater risk than other patients of surgically related AE per admission, 8.7 vs. 5.7% (p = .002, rr 1.54, 95% CI 1.18–2.00). This is primarily due to events termed ‘other operative complications’, 3.1 vs. 1.9%. Cancer patients experience twice the rate of medication-related AE per admission, 34 vs. 17% (p = .005, rr 2.03, 95% CI 1.23–2.91). Adjusted for length of stay and other demographic variables, cancer patients have a 58% higher risk for medication-related AEs per 1000 patient days, 2.6 vs. 1.6 (p = .045, rr 1.58, 95% CI 1.01–2.46) ().
Table 6. Comparing type of AEs per admission.
Discussion
Hospitalised cancer patients have a 39% greater risk of experiencing an AE compared to other patients, but this is due to older age, longer length of stay and surgery rather than the cancer itself. There is no difference in occurrence of AEs by type of cancer, but patients receiving treatment with curative intent and undergoing surgery have a higher rate of AEs.
Length of stay is the main risk factor for experiencing an AE, increasing the risk with 5.1% for each day spent in hospital. In our study, cancer patients stay 2.27 days longer in hospital, increasing the risk for AEs by 11.5%. Other studies have shown that there is a strong correlation between length of stay and rate of AEs [Citation7,Citation16]. The average length of stay in Norway for all hospitalised patients in 2013 was 5.6 days and 6.1 days for cancer patients [Citation17]. Our study correlates with findings for the overall hospital population, while our cancer patients are admitted two days longer than the national average. Increased rates of AEs can both be the cause for or a consequence of longer length of stay [Citation16,Citation18]. Our study was not designed to clarify this question.
A meta-analysis of AEs measured by the GTT, found an average of 29% of admissions with at least one AE and an average of 61 AEs per 1000 patient days [Citation3]. These average rates are higher than we found in our study, but comparing rates are difficult due to differences in study population and case mix. A Norwegian national GTT measurement shows that the total harm rate on average was 15.96% for the same time period [Citation19]. This is lower than our rate and could be due to the fact that Nordland Hospital Trust reviews seven times more patient records than other hospitals in Norway [Citation12]. Our results are consistent with findings from Denmark where similar rates of AEs were detected in cancer patients [Citation20].
The district hospital of Lofoten has a 30% lower rate of AE (13.4 per 1000 patient days). This is significantly lower than the Central Hospital of Bodø (17.7 per 1000 patient days) but not much lower than the Local Hospital of Vesterålen (14.6 per 1000 patients days). These findings correlate with the size of the hospitals and are most likely explained by the fact that the local hospitals perform less surgery and have a lower DRG index.
Admissions with AEs tell us what happens to the patients, while AEs per 1000 patient days adjusts for one important risk factor, and makes it more appropriate for monitoring over time. In addition, our data show that adjusting for other characteristics such as age, gender, type of admission and department further decreases the rate of AEs per 1000 patient days to 37.1 vs. 24.4 and 36.0 vs. 26.0 respectively. This implies that demographic characteristics significantly affect the rate of AEs. Demographic variables may vary and especially affect small sample sizes as recommended reviewed in the IHI GTT method. Not adjusting for demographic variables therefore raises concern about the GTT methods ability to detect real change when monitoring AEs even within an organisation.
The age of patients is a main risk factor for AEs [Citation7,Citation21]. Our results indicate that for every year increase in age, the risk of an AE increases by 1.3%. In our sample, cancer patients are 10.2 years older than other patients, increasing the risk by 13%. Age is also a strong determinant of cancer risk and an ageing population will increase the cancer rate per se. This implies that more patients will need cancer treatment, and thus escalate the burden on cancer care, and risk of AEs in our hospitals [Citation22].
Our results are consistent with other studies indicating that admission to a surgical department and having surgery increases the rate of AEs [Citation16,Citation23]. A majority of our cancer patients are admitted to surgical departments and 36% have surgery. Since surgery is the main curative treatment for cancer, this partly explains why receiving treatment with curative intent increase the risk for AEs.
In accordance with other GTT studies on cancer patients, cancer patients more often experience AEs related to hospital-acquired infections (lower respiratory infections and other infections), surgical complications and medication harm [Citation24,Citation25]. Adjusted for length of stay and other demographic variables, the only type of AE cancer patients experience more often is harm related to medication. Unfortunately, we do not know if these AEs relate to chemotherapy or other medications. The GTT method only registers if an AEs has occurred, and does not identify supplementary information such as type of medication, dosage or polypharmacy that could identify underlying causes and benefit further improvement work. Many of the categories are heterogeneous and need to be more specific to provide meaningful data for improvement in cancer care.
Our sample is representative for a general hospitalised population and shows that the majority of cancer patients receive surgery or ordinary medical treatment. Only 15% of the patients receive cancer related treatment such as chemotherapy or radiation. Our study shows that cancer in itself is not a risk factor for AEs, and hospitalised cancer patients seem to have the same general risk factors for AEs as other patients. Having cancer should therefore not affect the reliability of the GTT method when used in an ordinary in-hospital population. The fact that only 15% of hospitalised cancer patients receive medical or radiation related cancer treatments indicates that monitoring AEs related to these treatments preferably should be done in an ambulatory setting.
Limitations
The GTT as a method has limitations that most likely also apply to our study. The reliability of record reviewing is moderate to sustainable when done by a small group of reviewers [Citation9]. In our study, seven different teams (21 reviewers) did the review. Even though the teams had the same training, where fairly consistent and reviewed 960 records during the period, there is a possibility that their judgement of what is an AE, severity grading and classification could vary between the teams and deviate over time. Another GTT study in the health trust has shown substantial agreement between teams, but our study was not designed to look at inter-rated reliability [Citation12]. As a retrospective record review method, the GTT may have limitations regarding documentation bias, since reviewers must rely on information recorded in the patient charts. This could be avoided performing a real time observation study, but does not seem feasible to use as a method to measure AEs over time. Nonetheless, GTT is one of the few tools measuring AEs in health care, and is recommended for use in many organisations worldwide. Another limitation of our study is not adjusting for comorbidities, especially since the age difference between the groups is more than 10 years and cancer patients therefore could have more comorbidity.
Conclusions
Hospitalised cancer patients more often than other patients experience AEs, but this is due to older age, longer length of stay and surgery rather than the cancer itself. In addition, our study shows that demographic characteristics affect the rate of AEs, and raise reliability concerns regarding the GTT method’s ability to detect real change when monitoring AEs over time. When measuring AEs in a general hospitalised population, the GTT method seems just as reliable for cancer patients as other patients. Since only a small amount of hospitalised cancer patients receives medical or radiation related cancer treatments, we suggest that a method for measuring AEs in an outpatient cancer setting should be developed. Developing cancer specific categories for AEs would also be essential in order to provide meaningful data for improvement in cancer care.
Ethical considerations
The Regional Committee of Ethics in Norway has reviewed the study and categorised it as retrospective health record research, which does not require approval by the committee (2013/1823).
Acknowledgments
The authors would like to thank the GTT review teams at Nordland Hospital Trust, Tom Wilsgaard for advice on statistical analysis, Alexander Ringdal, Elisabeth Mentzoni and Marina Mineeva for help with data processing.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Vincent C, Taylor-Adams S, Stanhope N. Framework for analysing risk and safety in clinical medicine. BMJ. 1998;316:1154–1157.
- Nolan TW. System changes to improve patient safety. BMJ. 2000;320:771–773.
- Health Quality & Safety Commission New-Zealand. The global trigger tool: a review of the evidence (2016 edition) [Internet]. Weelington, NZ; 2016. Available from: www.hqsc.govt.nz
- Hannisdal E, Arianson H, Braut GS, et al. A risk analysis of cancer care in Norway: the top 16 patient safety hazards. Jt Comm J Qual Patient Saf. 2013;39:511–516.
- National Patient Safety Foundation. Free from Harm: Accelerating Patient Safety Improvement Fifteen Years after To Err Is Human. Boston, MA; 2015. Available from: www.npsf.org
- Doupi P, Svaar H, Bjørn B, et al. Use of the global trigger tool in patient safety improvement efforts: Nordic experiences. Cogn Technol Work. 2014;17:45–54.
- Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30:581–589.
- Schildmeijer K, Nilsson L, Arestedt K, et al. Assessment of adverse events in medical care: lack of consistency between experienced teams using the global trigger tool. BMJ Qual Saf. 2012;21:307–314.
- Hanskamp-Sebregts M, Zegers M, Vincent C, et al. Measurement of patient safety: a systematic review of the reliability and validity of adverse event detection with record review. BMJ Open. 2016;6:e011078.
- Mattsson TO, Knudsen JL, Lauritsen J, et al. Assessment of the global trigger tool to measure, monitor and evaluate patient safety in cancer patients: reliability concerns are raised. BMJ Qual Saf. 2013;22:571–579.
- Deilkås ET, Bukholm G, Lindstrøm JC, et al. Monitoring adverse events in Norwegian hospitals from 2010 to 2013. BMJ Open. 2015;5:e008576.
- Mevik K, Griffin FA, Hansen TE, et al. Does increasing the size of bi-weekly samples of records influence results when using the global trigger tool? An observational study of retrospective record reviews of two different sample sizes. BMJ Open. 2016;6:e010700.
- Norwegain Institute of Public health; The Knowledge Centre for the Health Services. Strukturert journal undersøkelse, ved bruk av Global Trigger Tool for å identifisere og måle forekomst av skader i helsetjenesten [Structured journal review, using the GTT method to identify and measure incidence of harm in health care]. Oslo; 2010.
- Griffin F, Resar R. IHI global trigger tool for measuring adverse events. second ed. IHI Innovation Series white paper; 2009. Available from: www.IHI.org
- Hartwig SC, Denger SD, Schneider PJ. Severity-indexed, incident report-based medication error-reporting program. AM J Hosp Pharm. 1991;48:2611–2616.
- Cihangir S, Borghans I, Hekkert K, et al. A pilot study on record reviewing with a priori patient selection. BMJ Open. 2013;3:e003034.
- Eurostat Statistics European Union. Hospital discharges and length of stay statistics – statistics explained. Webpage; 2015. Available from: http://ec.europa.eu/eurostat/statistics-explained/index.php/Hospital_discharges_and_length_of_stay_statistics
- Rutberg H, Borgstedt Risberg M, Sjödahl R, et al. Characterisations of adverse events detected in a university hospital: a 4-year study using the global trigger tool method. BMJ Open. 2014;4:e004879.
- Bjertnaes O, Deilkås ET, Skudal KE, et al. The association between patient-reported incidents in hospitals and estimated rates of patient harm. Int J Qual Health Care. 2015;27:26–30.
- Mattsson TO, Knudsen JL, Brixen K, et al. Does adding an appended oncology module to the global trigger tool increase its value?. Int J Qual Health Care. 2014;26:553–560.
- Hébert G, Netzer F, Ferrua M, et al. Evaluating iatrogenic prescribing: development of an oncology-focused trigger tool. Eur J Cancer. 2015;51:427–435.
- Cancer Registry of Norway. Cancer in Norway 2015 – cancer incidence, mortality, survival and prevalence in Norway. Oslo; 2016. Available from: www.kreftregisteret.no
- Kennerly DA, Kudyakov R, da Graca B, et al. Characterization of adverse events detected in a large health care delivery system using an enhanced global trigger tool over a five-year interval. Health Serv Res. 2014;49:1407–1425.
- Griffin FA, Classen DC. Detection of adverse events in surgical patients using the trigger tool approach. Qual Saf Health Care. 2008;17:253–258.
- Lipczak H, Knudsen JL, Nissen A. Safety hazards in cancer care: findings using three different methods. BMJ Qual Saf. 2011;20:1052–1056.