Background
Since the year 2000, several tyrosine kinase inhibitors (TKIs) have been introduced in the clinical setting to treat metastatic cancers. The TKIs sunitinib, pazopanib, axitinib and very recently, cabozantinib became available for patients with metastatic renal cell carcinoma (mRCC). Large differences in response, duration of progression free survival, toxicity, and quality of life between patients using TKIs are often observed [Citation1]. Therefore, individualizing TKI treatment should play a key role in the treatment of mRCC to offer patients survival benefit while preserving the quality of life.
Here, we describe a patient with mRCC with no response observed to the second, third and last line of treatment. However, finally a solution was found.
Case presentation
In our outpatient clinic, a 54-year-old male patient was diagnosed with locally advanced RCC in January 2013 (stage PT3aN1R1) with poor prognosis according to the memorial Sloan Kettering Cancer Center (MSKCC) criteria. Initially, a nephrectomy with lymphoid dissection was performed, but in July 2013 he developed lymph node metastases for which palliative treatment with sunitinib 50 mg once daily was started in the 4 weeks on/2 weeks off schedule. After 13 months of treatment, the patient developed severe peritoneal carcinomatosis with ascites. Consecutively, everolimus 10 mg daily and pazopanib 800 mg daily were initiated. Unfortunately, ascites drainages of ca. 11–13 L were still mandatory during both treatments and indicated clinical disease progression.
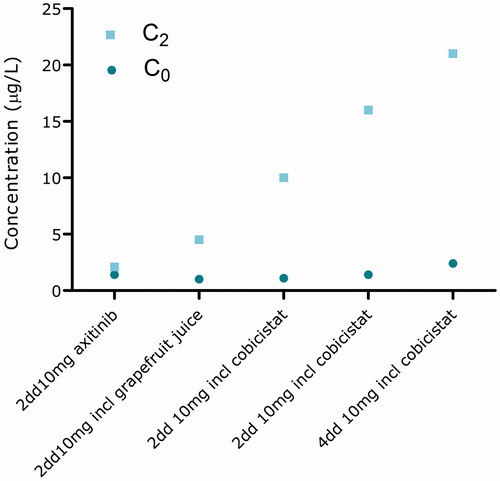
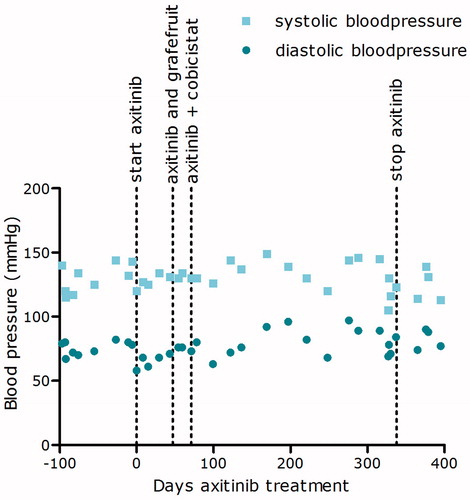
As a last treatment option, therapy was switched to axitinib 5 mg twice daily. Because of the absence of any axitinib toxicity, no appearance of hypertension and an increase in the amount of ascites, the dose was pragmatically increased to 10 mg twice daily after 2 weeks of therapy. At that time, oxycodone, nadroparin, and ranitidine were concomitantly used. However, even at this high dose still no toxicity or rise in blood pressure was seen. Axitinib pharmacokinetic was evaluated to assess drug exposure and uptake (). The axitinib trough level was 1.4 μg/L, which is below the threshold associated with efficacy (reference >5 μg/L) [Citation2]. The patient’s co-medication was monitored for drug–drug interactions and no drug–drug interactions were found. Subsequently, the pharmacogenetic profile showed no alterations in the cytochrome-P450 (CYP) CYP1A2, 2C19 enzymes. However, a polymorphism in CYP3A4 (*1A/*1B) was found.
To increase axitinib exposure, first grapefruit juice was added to inhibit intestinal CYP3A4 enzyme activity and enhance the uptake of axitinib. However, no significant improvement in plasma exposure was seen. In the meantime, another ascites drainage of 13 L was required indicating clinically progressive disease. Next, grapefruit juice was replaced by cobicistat 150 mg twice daily, a very potent and selective CYP3A4 inhibitor [Citation3]. After 4 weeks of concomitantly axitinib and cobicistat intake, blood pressure started to increase slightly (). The axitinib concentrations 2 h after intake (C2) increased, but surprisingly, axitinib trough levels remained below the favorable threshold of 5 μg/L [Citation2]. Therefore, the axitinib dose was further increased to 10 mg four times daily combined with cobicistat 150 mg four times daily. During the combined axitinib and cobicistat therapy, no ascites drainage was needed. The CT-scan showed a decrease in size of metastasis and hemoglobin and albumin levels normalized, indicating a therapeutic response that earlier could not be observed.
After 15 months of successful axitinib–cobicistat treatment, progressive disease was observed and the patient was switched back to sunitinib. Eventually, the patient died as a result of progressive disease 2 months after starting sunitinib therapy.
Discussion
Axitinib is primarily metabolized by CYP3A4 and to a lesser extent by CYP1A2, CYP2C19 and uridine diphosphate glucuronosyltransferase (UGT) 1A1. No pharmacologically active metabolites are formed [Citation4]. The pharmacogenetic screening showed a CYP3A4 polymorphism of *1A/*1B. The effect of this polymorphism on drug exposure is currently unclear. Tran et al. showed that patients with a *1A/*1B polymorphism had an increase in CYP3A4 enzyme expression resulting in a decreased docetaxel exposure [Citation5]. However, in other studies, no effect of this polymorphism in drug exposure was seen. If this patient had an increased CYP3A4 expression or functioning, an effect on sunitinib, everolimus and pazopanib exposure is expected since they are all primarily metabolized by CYP3A4 as well. Pazopanib trough level was retrospectively measured as part of a clinical trial and sunitinib trough level was measured at the second treatment initiation. Pazopanib trough level was 15 mg/L (normal values 24.6–30.9 mg/L) and the sum of sunitinib and the metabolite SU1266 trough level was 45 μg/L (normal values 62.4–84.9 μg/L) [Citation6]. Both trough levels where below normal values. This strengthens the hypothesis that this patient had an increased CYP3A4 activity resulting in reduced axitinib, pazopanib and sunitinib plasma exposure levels. Whether this is the result of the genetic polymorphism in CYP3A4 remains unknown.
To boost axitinib exposure, cobicistat was added to the treatment. Cobicistat inhibits the CYP3A4 enzyme present in the intestinal epithelium and thereby increases bioavailability. Furthermore, it reduces systemic metabolism by inhibiting hepatic CYP3A4 enzymes. Since intestinal CYP3A inhibition has the most profound effect on drug exposure, cobicistat was administered concomitantly with the axitinib to maximize the effect of CYP3A inhibition [Citation7].
In a study by Pithavala et al., the effect of ketoconazole, a potent CYP3A4 inhibitor, on axitinib exposure was investigated. A twofold increase on axitinib exposure was seen when axitinib was concomitantly ingested with ketoconazole. Cobicistat is a more potent 3A4 inhibitor than ketoconazole therefore a more than twofold increase in axitinib exposure was expected [Citation8].
Interestingly, during co-administration with cobicistat, axitinib trough levels did not significantly increase even though the C2 level did increase. The clinical response in the absence of sufficient trough levels can possibly be explained by the over-expression of CYP3A enzymes in tumor cells in patients with renal cell cancer [Citation9]. This could indicate that axitinib is not only metabolized systemically in the liver, but also intracellularly in tumor cells. Inhibiting CYP3A metabolism by adding cobicistat to axitinib therapy could potentially increase the intracellular axitinib concentration by inhibiting the intratumorcel CYP3A enzymes, leading to an increased intracellular exposure of axitinib resulting in a more effective treatment.
Here, we described an mRCC patient treated with cobicistat boosted axitinib treatment. This resulted in increased axitinib exposure and improved the quality of life by reduction of ascites. The patient responded to cobicistat boosted axitinib therapy for 15 months compared with 8.3 months, which is seen on average [Citation10]. Although our observations have to be explored further, we believe that boosting axitinib exposure with cobicistat may be a promising and cost-effective way to increase axitinib response in patients with treatment failure hypothetically due to sub-optimal exposure.
References
- Suttle AB, Ball HA, Molimard M, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111:1909–1916.
- Tsuchiya N, Igarashi R, Suzuki-Honma N, et al. Association of pharmacokinetics of axitinib with treatment outcome and adverse events in advanced renal cell carcinoma patients. JCO. 2015;33(suppl 7):abstr 506.
- Marzolini C, Gibbons S, Khoo S, et al. Cobicistat versus ritonavir boosting and differences in the drug–drug interaction profiles with co-medications. J Antimicrob Chemother. 2016;71:1755–1758.
- Zientek MA, Goosen TC, Tseng E, et al. In vitro kinetic characterization of axitinib metabolism. Drug Metab Dispos. 2016;44:102–114.
- Tran A, Jullien V, Alexandre J, et al. Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79:570–580.
- Yu H, Steeghs N, Nijenhuis CM, et al. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53:305–325.
- Lepist EI, Phan TK, Roy A, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56:5409–5413.
- Pithavala YK, Tong W, Mount J, et al. Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Invest New Drugs. 2012;30:273–281.
- Murray GI, McFadyen MC, Mitchell RT, et al. Cytochrome P450 CYP3A in human renal cell cancer. Br J Cancer. 1999;79:1836–1842.
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562.