Abstract
Purpose: To report the outcomes of patients with previously untreated cutaneous Merkel cell carcinoma (MCC) managed with curative intent.
Material and methods: Between December 1984 and August 2015, 59 patients with previously untreated cutaneous MCC were managed with curative intent with surgery and adjuvant radiotherapy (54 patients) or radiotherapy alone (5 patients) at the University of Florida. Primary sites included head and neck (45 patients), extremities (11 patients) and trunk (3 patients). Adjuvant chemotherapy was employed in 14 patients. Patients were staged according to the AJCC staging system: stage I, 25 patients; stage IIA, 7 patients; and, stage III, 27 patients. No patients had distant metastases.
Median follow-up for all patients was 3.2 years (range, 0.3–20.9 years). Median follow-up for survivors was 6.7 years (range, 1.6–20.9 years).
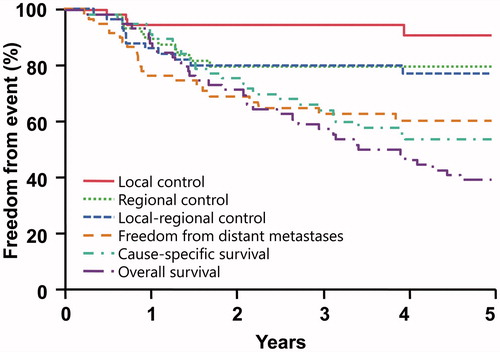
Results: The 5-year outcomes were as follows: local control, 91%; regional control, 79%; local–regional control, 77%; disease metastasis-free survival, 60%; cause-specific survival, 53%; and overall survival, 39%. The 5-year outcomes for patients with stage I–IIA versus stage-III disease were the following: local–regional control, 90% versus 57% (p = .0115); distant metastasis-free survival, 78% versus 36% (p = .0002); cause-specific survival, 68% versus 35% (p = .0050); and overall survival, 48% versus 27% (p = .0377). Local–regional recurrences occurred in 12 patients; no patients were successfully salvaged. Severe late complications were observed in four patients.
Conclusions: Although radiotherapy alone or combined with surgery results in a relatively high likelihood of local–regional control, the majority of recurrences are distant and approximately half of patients are cured. Patients with regional disease at diagnosis have significantly worse outcomes.
Introduction
Cutaneous Merkel cell carcinoma (MCC) is an uncommon neuroendocrine malignancy that is associated with the polyomavirus (MCPyV) in approximately 80% of patients [Citation1]. MCPyV-positive MCCs have an improved prognosis compared with MCPyV-negative MCCs [Citation1]. MCCs that are MCPyV negative have a high mutation rate and may respond to immunotherapy using programed death-1 and programed death ligand-1 blocking antibodies [Citation1]. MCC may be confused histologically both with other cutaneous malignancies and with other neuroendocrine carcinomas, including small cell lung carcinoma [Citation2,Citation3]. It is key to secure the correct diagnosis, because MCC has a high risk of local–regional recurrence after surgery alone and is not as likely to exhibit hematogenous dissemination and respond to cytotoxic chemotherapy as small cell neuroendocrine carcinoma. Although distant metastases appear after treatment in almost half of patients, it is important to maximize local–regional control to optimize the likelihood of cure and quality of life.
Because MCC is a relatively rare malignancy, most institutional experiences have limited numbers of patients. The aim of this paper is to update the outcomes of patients treated with radiotherapy (RT) alone or combined with surgery at our institution.
Material and methods
Between December 1984 and August 2015, 59 patients were treated with either surgery and adjuvant RT (54 patients) or RT alone (5 patients) with curative intent at the University of Florida. There were 45 men and 14 women; the median age was 70 years (range, 38 to >89 years). All patients were white. Primary sites were: head and neck, 45 patients (76%); extremities, 11 patients (19%); and trunk, 3 patients (5%). The diagnostic evaluation included physical examination, review of outside pathology, computerized tomography of the regional nodes and chest and, more recently, positron emission tomography [Citation4,Citation5]. MCPyV status was not assessed at diagnosis. Sentinel lymph node biopsy (SLNB) was not routinely performed, because our philosophy has been to irradiate the regional nodes regardless of the results of SLNB. Patients were clinically staged according to the American Joint Committee on Cancer staging system [Citation6]. The T-stage distribution was: T0, 3 patients; T1, 39 patients; T2, 15 patients; and T3, 2 patients. The N-stage distribution was: N0, 32 patients; N1, 21 patients; and N3, 6 patients. The overall stage distribution was: I, 25 patients; IIA, 7 patients; and III, 27 patients.
Surgery was part of treatment in 56 patients (95%) and included resection of the primary site in 35 patients (59%), regional node dissection in two patients (3%), and both in 19 patients (32%). A regional node dissection was performed in 6 of 33 patients (18%) with stage-I disease and 15 of 26 patients (58%) with stage-IIA and stage-III disease. Resection margins were: negative (R0), 42 patients (75%); microscopically positive (RI), 8 patients (14%); and grossly positively (R2), 6 patients (11%).
Adjuvant RT was administered postoperatively in 50 patients (85%) and preoperatively in 4 patients (7%). The median RT dose was 60 Gy (range: 30–74 Gy). The majority of patients received approximately 50 Gy to undissected cN0 regional nodes, 60 Gy postoperatively for R0 margins, 66 Gy for R1 margins and 70 Gy for gross disease at approximately 2 Gy per once-daily fraction.
Fifty patients (85%) were treated with once-daily fractionation; the remaining nine were treated with twice-daily fractionation (8) or concomitant boost (1). Three patients (5%) received 30 Gy in five twice-weekly fractions because of logistical difficulties that precluded conventional fractionation. Regional node RT was employed in 31 of 33 patients (94%) with stage-I disease and in 24 of 26 patients (92%) with stage-II disease.
Adjuvant chemotherapy was employed in 14 patients (24%) including 12 of 26 patients (46%) with stage-II disease. The agents administered included etoposide (VP-16) (11 patients), cisplatin (8 patients) and carboplatin (6 patients). The number of cycles ranged from 1 to 7.
The median follow-up for all patients was 3.2 years (range: 0.3–20.9 years) and for survivors was 6.7 years (range: 1.6–20.9 years). No patients were lost to follow up.
SAS and JMP software (SAS Institute, Cary, NC) was used for all statistical computations. The Kaplan–Meier product-limit method was used to provide estimates of local control, regional control, local–regional control, distant metastasis-free survival, cause-specific survival and overall survival. Complications were coded as severe if they necessitated hospitalization, surgical intervention, or resulted in death [Citation7].
Results
The five-year outcomes are summarized in and . Local–regional control was achieved in 47 of 59 patients (80%). Five patients were treated for unresected local or regional disease with RT alone and three of five were local–regionally controlled.
Table 1. Five-year outcomes.
Local–regional recurrences occurred in 12 patients; no patients were successfully salvaged. Distant metastases were observed in 22 patients. Distant metastases developed in 9 of 14 patients (64%) who received adjuvant chemotherapy and 13 of 45 patients (29%) who did not receive adjuvant chemotherapy (p = .0263). One patient had a lesion excised from his wrist and had 20 of 23 positive axillary nodes dissected. He received postoperative RT and adjuvant chemotherapy. He developed a solitary 7 cm metastasis in the medial thigh 6 months later that was treated with surgery and postoperative RT. He remains continuously disease free 6 years following completion of his second course of RT.
Severe complications occurred in four patients (7%) and included: a bone exposure in the oral cavity (1 patient), hospitalization during RT for dehydration (2 patients) and hospitalization for nausea and dehydration (1 patient). All three patients who were hospitalized during RT also received chemotherapy; one of three did not complete RT as planned.
Discussion
There are questions pertaining to the optimal management of patients with MCC including the efficacy of SLNB, the impact of adjuvant RT after surgery, the outcomes after RT alone, the optimal RT dose and the role of adjuvant chemotherapy [Citation8–22].
Sentinel lymph node biopsy
Grotz et al. reported on 111 patients who underwent a negative SLNB between 1995 and 2011 at the Mayo Clinic (Rochester, MN); 13 patients (12%) received adjuvant RT to the regional nodes [Citation17]. The 5-year regional recurrence rate was 17%. Gunaratine et al. reported on a literature review of 721 patients including 29 from the authors' institution who underwent SLNB and were treated between 1997 and 2015 [Citation21]. The incidence of positive SLNB was 30%. Regional recurrences were observed in 17% of those with a negative SLNB. The addition of adjuvant nodal RT after a negative SLNB did not significantly impact regional control (p = .31).
Adjuvant radiotherapy
Strom et al. reported on 171 patients treated with surgery with or without adjuvant postoperative RT between 1994 and 2012 at the Moffitt Cancer Center (Tampa, FL); all had a pathologic nodal evaluation [Citation16]. Median follow-up was 33 months. The addition of adjuvant RT was associated with improvement in the following 3-year outcomes: local control, 91% versus 77% (p = .01); local–regional control, 80% versus 59% (p = .004); disease-free survival, 57% versus 30% (p < .001); and overall survival, 73% versus 66% (p = .02). Multivariate analysis revealed that RT was associated with improved local control (p < .001), local–regional control (p < .001), disease-free survival (p = .001), overall survival (p = .03) and disease-specific survival (p = .001). Bhatia et al. reported on 6908 patients with American Joint Committee on Cancer stage I–III lesions included in the National Cancer Data Base between 1996 and 2008. (Bhatia) Multivariate analysis revealed that surgery and RT resulted in improved overall survival for patients with stage-I and stage-II MCCs compared with surgery alone (p < .001 for both). The addition of adjuvant RT and/or chemotherapy did not significantly impact overall survival for patients with regional nodal metastases (stage III).
Definitive radiotherapy
Veness and Howle reported on 41 patients treated at Westmead Hospital (Sydney) with definitive RT alone between 1993 and 2013; 59% were treated at initial diagnosis and the remainder at relapse [Citation22]. Median dose to the primary site was 51 Gy (range: 20–63 Gy) and median dose to the nodes was 50 Gy (range: 20–64 Gy). Median dose per fraction was 2 Gy (range: 2–5 Gy). Median lesion size was 5 cm (range: 0.5–13 cm). Median follow-up was 39 months. The in-field control rate was 85%; the 5-year overall survival rate was 40%. Harrington and Kwan reported on 57 patients treated with RT for macroscopic local–regional disease at the British Columbia Cancer Agency (Vancouver) between 1979 and 2007 [Citation23]. The two-year control rate for macroscopic disease was 82%. The five-year outcomes were: relapse-free survival, 57%; cancer-specific survival, 68%; and overall survival, 39%. Multivariate analysis revealed that RT doses >50 Gy were associated with improved cancer-specific survival. Gunaratne et al. reported on a literature search that included 23 studies with 264 patients treated with definitive RT for macroscopic disease [Citation19]. The mean doses to the primary site and regional nodes were 48.7 Gy and 49.4 Gy, respectively. The cumulative in-field recurrence rate rates were as follows: overall, 12%; primary site, 8%; and regional nodes, 17%. There was no significant relationship between RT dose and in-field disease control. Patel et al. reported on 1625 patients with head and neck MCCs included in the National Cancer Data Base between 1998 and 2011 [Citation12]. All patients were treated with surgery and RT with doses ranging between 30 and 70 Gy. Median follow-up was 33.5 months. The three-year overall survival rates were 30 to <50 Gy, 49%; 50–55 Gy, 70%; and >55 Gy to 70 Gy, 59%. Patients who received 50–55 Gy had significantly better overall survival rates than those who received higher or lower doses (p < .001). A caveat is that selection bias may have impacted outcome.
Adjuvant chemotherapy
Chen et al. reported on 4815 patients with head and neck MCC included in the National Cancer Data Base between 1998 and 2011 [Citation24]. Patients were treated with surgery alone or combined with adjuvant RT or chemoradiation (CRT). Postoperative RT and CRT were associated with significantly improved survival compared with surgery alone. Adjuvant CRT was associated with improved overall survival compared with adjuvant RT for positive margins, male patients and for those with tumor size 3 cm or larger.
Conclusions
The optimal treatment for patients with local–regional MCC is likely surgery and postoperative RT. SLNB is useful for deciding whether or not to electively irradiate the regional nodes in cN0 patients. However, the rate of regional relapse after a negative SLNB is relatively high and, if the plan is to electively irradiate the nodes regardless of the results of the SLNB, the procedure may be eliminated. RT alone is a reasonable alternative for patients in whom surgery is not a treatment option due to medical comorbidities or a suboptimal functional or cosmetic outcome. Although the authors’ bias is to err on the side of higher RT doses, 50–55 Gy may be adequate, even for macroscopic disease. Patients with poor prognostic factors such as positive margins and more advanced disease may benefit from adjuvant chemotherapy. The optimal agents are likely those employed for small cell lung cancers and include cisplatin, etoposide and carboplatin. Patients who present with distant metastases may benefit from targeted agents such as pembrolizumab and atezolizumab [Citation1].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Terheyden P, Becker JC. New developments in the biology and the treatment of metastatic Merkel cell carcinoma. Curr Opin Oncol. 2017. DOI:10.1097/CCO.0000000000000363
- Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–110.
- Mendenhall WM, Kirwan JM, Morris CG, et al. Cutaneous Merkel cell carcinoma. Am J Otolaryngol. 2012;33:88–92.
- Byrne K, Siva S, Chait L, et al. 15-Year experience of 18F-FDG PET imaging in response assessment and restaging after definitive treatment of Merkel cell carcinoma. J Nucl Med. 2015;56:1328–1333.
- Llombart B, Kindem S, Chust M. Merkel cell carcinoma: an update of key imaging techniques, prognostic factors, treatment, and follow-up. Actas Dermosifiliogr. 2017;108:98–107.
- Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. New York, NY: Springer; 2017.
- Taylor JM, Mendenhall WM, Lavey RS. Dose, time, and fraction size issues for late effects in head and neck cancers. Int J Radiat Oncol Biol Phys. 1992;22:3–11.
- Clark JR, Veness MJ, Gilbert R, et al. Merkel cell carcinoma of the head and neck: is adjuvant radiotherapy necessary? Head Neck. 2007;29:249–257.
- Foote M, Harvey J, Porceddu S, et al. Effect of radiotherapy dose and volume on relapse in Merkel cell cancer of the skin. Int J Radiat Oncol Biol Phys. 2010;77:677–684.
- Sexton KW, Poteet SP, Hill JB, et al. Adjuvant radiation therapy increases disease-free survival in stage IB Merkel cell carcinoma. Ann Plast Surg. 2014;73:531–534.
- Harrington C, Kwan W. Radiotherapy and conservative surgery in the locoregional management of Merkel cell carcinoma: The British Columbia Cancer Agency Experience. Ann Surg Oncol. 2016;23:573–578.
- Patel SA, Qureshi MM, Mak KS, et al. Impact of total radiotherapy dose on survival for head and neck Merkel cell carcinoma after resection. Head Neck. 2017;39:1371–1377.
- Bishop AJ, Garden AS, Gunn GB, et al. Merkel cell carcinoma of the head and neck: favorable outcomes with radiotherapy. Head Neck. 2016;38(Suppl 1):E452–E458.
- Bhatia S, Storer BE, Iyer JG, et al. Adjuvant radiation therapy and chemotherapy in Merkel cell carcinoma: survival analyses of 6908 cases from the National Cancer Data Base. J Natl Cancer Inst. 2016;108. DOI:10.1093/jnci/djw042
- Strom T, Naghavi AO, Messina JL, et al. Improved local and regional control with radiotherapy for Merkel cell carcinoma of the head and neck. Head Neck. 2017;39:48–55.
- Strom T, Carr M, Zager JS, et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann Surg Oncol. 2016;23:3572–3578.
- Grotz TE, Joseph RW, Pockaj BA, et al. Negative sentinel lymph node biopsy in Merkel cell carcinoma is associated with a low risk of same-nodal-basin recurrences. Ann Surg Oncol. 2015;22:4060–4066.
- Veness M, Foote M, Gebski V, et al. The role of radiotherapy alone in patients with merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78:703–709.
- Gunaratne DA, Howle JR, Veness MJ. Definitive radiotherapy for Merkel cell carcinoma confers clinically meaningful in-field locoregional control: a review and analysis of the literature. J Am Acad Dermatol. 2017;77:142–148.
- Poulsen MG, Rischin D, Porter I, et al. Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys. 2006;64:114–119.
- Gunaratne DA, Howle JR, Veness MJ. Sentinel lymph node biopsy in Merkel cell carcinoma: a 15-year institutional experience and statistical analysis of 721 reported cases. Br J Dermatol. 2016;174:273–281.
- Veness M, Howle J. Radiotherapy alone in patients with Merkel cell carcinoma: the Westmead Hospital experience of 41 patients. Australas J Dermatol. 2015;56:19–24.
- Harrington C, Kwan W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann Surg Oncol. 2014;21:3401–3405.
- Chen MM, Roman SA, Sosa JA, et al. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: an analysis of 4815 patients. JAMA Otolaryngol Head Neck Surg. 2015;141:137–141.