Abstract
Background: Phase II trials suggested that survival rates for locally advanced lung cancer could be increased by radiotherapy dose escalation. However, results of the phase III RTOG 0617 trial illustrated an imminent risk of treatment-related death. This could be thwarted with strict constraints to organs at risk (OARs) and control of the delivered dose. This study investigates the impact of anatomical changes during radiotherapy on escalated dose distributions used in the Danish NARLAL2 dose escalation trial.
Material and methods: The phase III NARLAL2 trial randomizes patients between a standard and an escalated treatment plan. In the escalated arm, mean doses up to 95 Gy/33 fractions (tumour) and 74 Gy/33 fractions (lymph nodes) are delivered to the most 18fluorodeoxyglucose-positron emission tomography (18FDG PET) active regions. The dose distributions are limited by strict constraints to OARs. For a group of 27 patients, a surveillance scan (sCT) was acquired at fraction 11. The original-escalated treatment plans were recalculated on the sCTs and the impact of inter-fractional changes evaluated.
Results: A total of 13 patients (48%) had overdosage of least one OAR. Constraints for the oesophagus, trachea and aorta were violated in 26% of the patients. No overdosage was seen for heart or bronchi. For the connective tissue (all tissue in the mediastinum not identified as OAR or tumour) overdosage was seen in 41% of the patients and for the chest wall in 30% of the patients. The main reason for overdosage was tumour shrinkage.
Conclusions: Anatomical changes during radiotherapy caused one or more OAR constraint violations for approximately half of the patient cohort. The main cause was tumour shrinkage. For lung cancer radiotherapy dose escalation trials, we recommend incorporation of adaptive radiotherapy strategies.
Introduction
To date, there exist no satisfactory treatment options for locally advanced lung cancer [Citation1]. Several phase II dose escalation studies point to a significant gain in local control [Citation2,Citation3]. However, in the phase III dose escalation trial RTOG 0617 [Citation4], a decrease in overall survival with escalation dose was observed. The initial multivariate analysis indicated that an explanation could be found in the dose to the heart [Citation4], but other reasons such as the prolonged treatment time in the escalated arm and greater survival for patients treated with IMRT have also been suggested [Citation5,Citation6]. For standard doses of 60–66 Gy, the dose-limiting organs at risk (OARs) are heart, lungs and oesophagus. This is also the case for dose escalation [Citation7–9] with the addition of mediastinal structures [Citation10,Citation11]. With intensity-modulated radiotherapy (IMRT), the dose distribution can be shaped according to dose prescription and normal tissue constraints [Citation12]. However, this only ensures pretreatment compliance with the constraints. Unfortunately, anatomical changes occur frequently for lung cancer patients. These changes can modify the dose distribution significantly [Citation13–15]. Using adaptive radiotherapy (ART), the dose distribution can be restored by replanning in case of any significant anatomical changes. For ART at the current standard dose, the main concern has been target coverage [Citation13–15]. In the case of dose escalation, anatomical changes may not only compromise target coverage, but could also result in critical overdosage of OARs.
The Danish NARLAL2 dose escalation trial is a randomized phase III trial. (NCT02354374) [Citation16]. Patients are randomized to either a standard homogeneous treatment plan of 66 Gy in 33 fractions or an experimental plan. In the experimental plan, the dose is heterogeneously escalated in a sub-volume of the tumour and malignant lymph nodes. This heterogeneous dose escalation is driven by high-18fluorodeoxyglucose-positron emission tomography (18FDG-PET) uptake volumes defined by a pretreatment 18FDG-PET scan. This way, similar normal-tissue doses can be achieved in both treatment arms, while the smaller high-18FDG-PET uptake volumes can be treated to high doses in the dose-escalated arm. A shift of these high-dose regions due to anatomical changes during radiotherapy can potentially become hazardous. Therefore, the present study investigates the impact of anatomical changes during radiotherapy on escalated dose distributions from the Danish NARLAL2 dose escalation trial.
Material and methods
The design of the NARLAL2 trial is illustrated in and described in detail in the following sections.
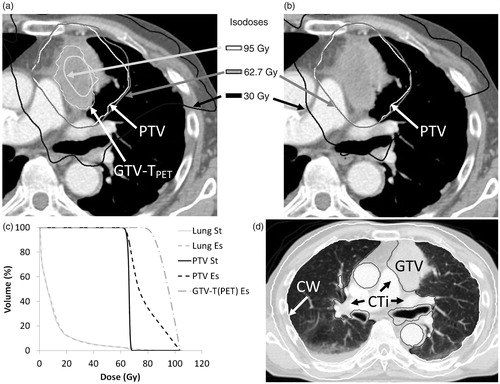
Figure 1. A NARLAL2-escalated plan (a) and a standard plan (b). PTV is shown for both (a) and (b), whereas GTV-TPET is only shown for the escalated plan. Comparison of the DVH parameters (c) shows that MLD remains virtually unchanged (MLDstandard =10.1 Gy and MLDescalated =10.4 Gy). Panel (d) illustrates the delineation of chest wall (CW, white) and connective tissue (CTi, black). The CTi includes all mediastinal tissue not otherwise defined as OAR or GTV. In the present image, the OAR were aorta, bronchi and oesophagus.
Pretreatment image acquisition and contouring
The patients were positioned with both arms above the head in a standard or an individualized immobilization device. For all patients, a free-breathing 18FDG planning PET/CT scan was acquired. In the same imaging session, a time-resolved 4D planning-CT scan was acquired with IV contrast. The mid-ventilation (mv) phase was selected for delineation and treatment planning (pCT) [Citation17]. The gross tumour volumes of the tumour (GTV-T) and the lymph nodes (GTV-N) were delineated using the maximum intensity projection, thus accounting for respiratory tumour motion [Citation18]. The lymph nodes were delineated separately to enable volume measurement of each malignant lymph node volume, and merged into the GTV-N. All nodes being PET positive or enlarged on CT were included. The GTVs were expanded to clinical target volumes (CTVs) by an isotropic 5 mm margin and corrected for invasion into bones and large blood vessels. For the CTV-T, the planning target volume (PTV) margin was 4 mm in the transversal plane and 5 mm in the longitudinal plane. A margin of 9 mm transversal and 10 mm longitudinal was used for CTV-N, in accordance to the reference [Citation15].
The following OARs were delineated: spinal cord, whole heart including pericardium, lungs, oesophagus, trachea, bronchi, aorta, connective tissue (CTi) and chest wall [Citation19,Citation20]. The solid part of the GTV was excluded from the lung delineation. The bronchi were delineated from 2 cm above the carina to the second division. The CTi was defined as all tissue located between the lungs, excluding the GTVs and remaining OARs, and the chest wall as a 2 cm wall around lung and mediastinum, see .
Definition of escalation volume
For each tumour and lymph node GTV, a separate dose escalation volume (GTV-XPET) was identified on the PET scan. It was defined as all pixels with standard uptake values (SUVs) above 50% of the SUVpeak within the GTV. SUVpeak was defined as the mean SUV in the 1 cm3 tissue volume with the highest SUV [Citation21]. If the 50% of SUVpeak cut-off yielded volumes below 4 cm3 or 30% of the GTV volume, 40% of SUVpeak or 30% of SUVpeak were used instead. For primary tumours and satellites with GTV <4 cm3, GTV-TPET was identical to the GTV-T. For lymph nodes <4 cm3, no dose escalation was performed. All escalation volumes were generated in MIM Vista (MIM Software, USA).
Treatment planning
All patients were treated using an IMRT technique with 5-8 beams of which maximally 2 were non-coplanar. Photon energy of 6 MV was chosen and anisotropic analytical algorithm (AAA) [Citation22] was used for dose calculation in the Eclipse treatment planning system (Varian Medical Systems, USA). In the standard arm, PTV was covered homogeneously by 66 Gy, with maximum and minimum allowed doses of 110% and 95%. In the experimental arm, PTV was covered with minimum 95% of 66 Gy as well. However, the dose was heterogeneously escalated to mean doses up to 95 Gy in the GTV-TPET and up to 74 Gy in GTV-NPET. There was no constraint on the maximum dose. The dose distribution was limited only by the strict OAR dose constraints listed in .
Table 1. Dose constraints applied to OARs.
The NARLAL2 protocol requires both plans to be approved for treatment before randomization. The mean lung dose (MLD) must be equal within ±1 Gy and the volume of the lungs receiving 20 Gy (V20 Gy) within 2% points. In the current study, only the escalated plans were analysed regardless of the randomization results. Thus, this study has no influence on the outcome of the trial.
sCT and re-delineation
For all patients, a surveillance 4D-CT scan was routinely acquired approximately at fraction 10. The mv phase was selected (sCT) and targets and OARs transferred from the pCT in both a rigid and a daemons-based deformable transfer (SmartAdapt, Varian Medical Systems, USA). Targets and OARs were manually corrected by an experienced radiation oncologist. While the GTV-T was adapted to the shrinkage of the tumour (sGTV-T), the intention was to maintain the size of the pretreatment CTV-T. The GTV-XPET volumes were rigidly transferred based on soft tissue GTV-T or GTV-N matches. Any parts of the GTV-TPET reaching outside sGTV-T were removed.
Evaluation of the effect of anatomical changes on OAR dose
The original treatment plan was transferred to the sCT through a GTV-T soft-tissue 4D rigid registration (3D-translational shifts and couch rotation). The plans and dose volume histograms were re-calculated for all target and OAR volumes using MLC movement and monitor units from the original plan. From this, median changes of PTV coverage (V95%) and of mean doses to GTV-TPET and GTV-NPET were found. Further, median changes of MLD and mean heart dose (MHD) were calculated. An increase in MLD or MHD of 1 Gy was here defined as overdosage. Compliance to OAR maximum dose constraints () was examined. To identify the degree of overdosage, additional dose metrics (D1cm3<74 Gy for oesophagus, D1cm3<78 Gy for heart, aorta, trachea, bronchi, CTi and chest wall) were investigated. Anatomical changes were identified visually and assigned to five categories: tumour shrinkage (TS), node shrinkage (NS), atelectasis (A), tumour-node shift (T-N) and positioning (P).
ART and clinical setup strategy
When overdosage of OARs was found on the routine sCT scan, adaptation was performed during the treatment course for all patients, regardless of the randomization result. Apart from the sCT at fraction 10, 3D cone-beam CT (CBCT) scans were acquired pretreatment for daily image-guided patient setup. The CBCT scan was automatically registered to the pCT based on a GTV-T soft-tissue match [Citation15,Citation23]. The registration was evaluated by the radiation therapists and the resulting 3D-translational shifts and couch rotation were performed. If systematic deviations in tumour or lymph node position were above a preset limit, adaptive replanning was performed as well [Citation15]. To avoid bias, these patient individual replanning CTs were not included in the current study.
Patients
A total of 31 patients were enrolled in the trial between April 2015 and April 2017 in our department. Due to clinical or technical issues, 4 patients had no sCT scan and were excluded from this study. The study group consisted of 11 females and 16 males, with a median age of 68 years (range 39–78 years). A total of 16 patients received cisplatin and Vinorelbine concomitant with radiotherapy [Citation24]. A total of 11 patients received only Vinorelbine concomitant with radiotherapy. The clinical stage distribution was 3 with stage IIB, 14 with stage IIIA and 10 with stage IIIB.
Results
Target coverage and dose to OARs for initialdose-escalated plan
The median [min;max] volumes of the total GTVmv was 93.3 cm3 [5.5;256.9 cm3] and the PTV was 381.9 cm3 [138.3;768.1 cm3]. The median PTV coverage was 99.5% [96.9;100%]. All patients had biopsy-verified tumour involvement. The median volume of GTV-TPET was 20.4 cm3 [2.0;124.5 cm3]. This volume was heterogeneously escalated to a median mean dose of 94.0 Gy [85.9;95.0 Gy]. Of the 27 patients, 21 had involvement of the lymph nodes. In total, 55 nodes were delineated but only 16 nodes (11 patients) were large enough to qualify for dose escalation (>4 cm3). The median volume of GTV-NPET was 18.5 cm3 [4.3;36.1 cm3] and the median mean dose was 73.3 Gy [72.3;73.9 Gy].
The median MLD was 14.0 Gy [6.7;18.6 Gy] and the median MHD was 2.5 Gy [0.3;18.8]. Only 6 patients had MHD >5 Gy. All OARs maximum dose constraints were met in the initial dose-escalated plans.
Target coverage and dose to OARs as recalculated on sCT
The median number of fractions treated before sCT was 11 [10;14]. The median change in PTV coverage was −1.3% [−9.4;0.2%]. The minimum coverage observed was 90.2% and for 4 patients the coverage decreased more than 5%. This was a result of a shift between tumour and lymph nodes (3 patients) or change in respiratory motion and deformation of the tumour on the sCT scan (1 patient). The mean doses to GTV-TPET and GTV-NPET were nearly unchanged with a median increase of 0.2 Gy [−2.2;2.8] and 0.3 Gy [−0.9;1.1], respectively. Only in 2 patients, a decrease in GTV-TPET mean dose of more than 1 Gy was observed.
The median increase in MLD was 0.2 Gy [−1.2;3.2 Gy]. In 5 patients, MLD increased more than 1 Gy. In these patients, GTV-T shrinkage between 19.1 and 106.0 cm3 were seen. There was no change in MHD (median 0.0 Gy [−1.2;2.2 Gy]). In 3 patients, MHD increased more than 1 Gy. However, for the 6 patients with MHD >5 Gy on pCT, the change was less than 1 Gy on sCT.
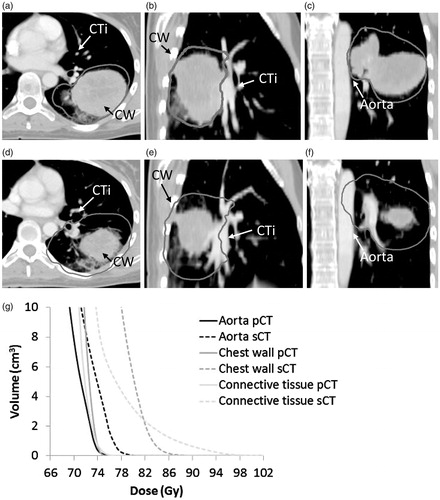
A total of 13 patients (48%) showed overdosage of at least one OAR on sCT, i.e., D1cm3 > 74 Gy (heart, aorta, trachea, bronchi, CTi and chest wall) or D1cm3 > 70 Gy (oesophagus), see . The maximum dose to oesophagus, trachea, aorta and bronchi was increased for 26% of the patients during the radiotherapy course and exceeded the constraints as defined by the protocol. For the bronchi, heart and spinal cord, the maximum dose constraints were maintained for all patients. For the CTi, 11 patients presented a D1cm3 above 74 Gy. In 4 of these patients, D1cm3 > 78 Gy was also exceeded. For the chest wall, although 8 patients exceeded the 74 Gy constraint, only 1 patient presented a D1cm3 of more than 78 Gy. For trachea and aorta, D1cm3 was less than 78 Gy and for oesophagus D1cm3 was less than 74 Gy in all patients. Shrinkage of the primary tumour was identified as the main reason for OAR overdosage (10 patients). While the median tumour shrinkage in all patients was 8.1 cm3 [−9.3;101.6], for these 10 patients, it was 34.6 cm3 [15.3;101.6 cm3]. shows a large tumour burden (patient 27), which has distorted the oesophagus and trachea. Escalation was limited by these OARs, resulting in a mean dose to GTV-TPET of 85.9 Gy and a steep dose gradient. Subsequent shrinkage of the tumour resulted in relaxation of the oesophagus and trachea, moving them into the high-dose region. presents the maximal tumour shrinkage observed in this study (101.6 cm3, patient 11). It resulted in massive overdosage of the chest wall, the CTi and the aorta. Changes in atelectasis and tumour-node shifts also resulted in overdosage of OARs. In 1 patient (patient 12), a shift between tumour and lymph nodes pulled oesophagus, aorta and CTi into the high-dose region when the soft tissue match was performed. Change in position and volume of an atelectasis at the tumour site lead to overdosage of the CTi in 1 patient (patient 22). Finally, changes in patient positioning gave rise to overdosage of the chest wall in 1 patient (patient 1).
Figure 2. Violated maximum OAR constraints for all patients in the study group individually. The absolute volume receiving 70/74 Gy is shown for each organ. For patient 11, 42 cm3 of aorta, CTi and chest wall received doses above 74 Gy. The reason for overdosage is indicated. TS: tumour shrinkage; NS: node shrinkage; A: atelectasis; T-N: tumour-node shift; P: positioning.
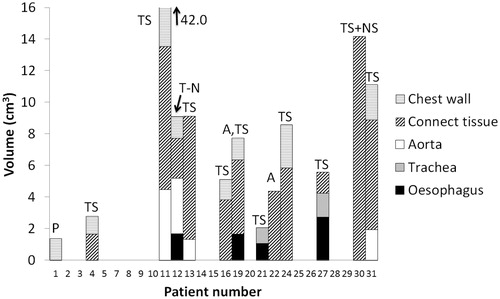
Figure 3. Overdosage of oesophagus (D1cm3 > 70 Gy) and trachea (D1cm3 > 74 Gy) due to tumour shrinkage between pCT (a) and sCT (b). The corresponding DVH’s for the oesophagus and trachea on pCT (full line) and sCT (dashed line) (c).
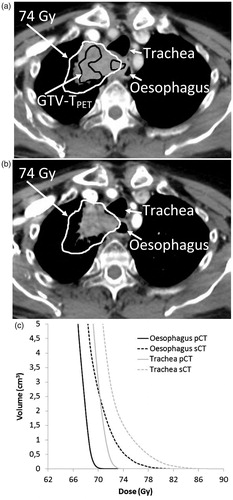
Figure 4. The largest tumour shrinkage observed in this study (101.6 cm3). Panel (a)–(c): Tumour at pCT. Panel (d)–(f): tumour at sCT. The 74 Gy isodose (dark gray), the aorta, the chest wall (CW) and the CTi are shown. Panel (g) The corresponding DVH’s for aorta, chest wall and CTi on pCT(full line) and sCT (dashed line).
Discussion
Despite the discouraging results of RTOG0617, the interest in non-small cell lung cancer (NSCLC) dose escalation studies has not diminished. It has been suggested that OAR toxicities may be part of the reason for the reduced overall survival in the high-dose arm [Citation4]. The RTOG0617 data analysis has been focused on pretreatment dose distributions without consideration of possible dose distribution changes during radiotherapy. The current study shows that pretreatment dose distributions can be altered substantially if anatomical changes occur during radiotherapy. This can lead to serious overdosage of OARs. Tumour shrinkage was identified as the primary cause of overdosage.
From studies of patients treated to standard dose, we know that approximately one-third of all NSCLC patients experience significant tumour shrinkage during radiotherapy [Citation14,Citation25]. Furthermore, differential motion between tumour and lymph nodes and change in atelectasis are also frequently observed [Citation13,Citation14,Citation23–26]. For dose escalation trials, NSCLC patients should therefore be monitored with soft tissue imaging on a regular basis. If necessary, compliance with OAR constraints should be restored by treatment plan adaption. Without adaption, approximately half of the patients in our study population would have received OAR doses above the NARLAL2 trial constraints.
Overdosage of the CTi was observed in 41% of the patients, whereof one patient reached doses above 74 Gy to a volume of 14.2 cm3. Although no clinical dose constraint recommendations exist for the CTi, both the NARLAL2 and the PET boost escalation trial [Citation27] restrict the dose to the mediastinum as a precaution. No mediastinal grade 4 or 5 toxicities have been reported in studies delivering 74 Gy to the whole tumour (including mediastinal targets) [Citation4,Citation28]. When performing heterogeneous dose escalation, steep gradients exist and very high-dose regions may move into mediastinal tissue. No clinical data are reported for dose levels above 74 Gy and thus, it may potentially be hazardous to deliver heterogeneous dose distributions in the mediastinum. Extensive constraint violations are observed for the chest wall as well. In one patient, 28.4 cm3 reached doses above 74 Gy. Again there are no clinical constraint recommendations from the standard NSCLC treatment regime. However, symptomatic rib fractures are of concern in lung stereotactic body radiation therapy (SBRT) [Citation29]. Also included in the chest wall structure, is the part of the vertebra adjacent to the lung. With the standard NSCLC treatment, vertebra fractures have been reported in 8% of the patients [Citation30].
In approximately one fourth of the patients, the maximum dose to oesophagus, trachea, aorta and bronchi were increased during the radiotherapy course and exceeded the constraints as defined by the protocol. In the NARLAL2 trial, no large hotspots receiving more than 70 Gy (oesophagus) or 74 Gy (trachea, bronchi, heart and aorta) were allowed during 33 fractions of RT. This corresponds to 71 Gy and 78 Gy in 2 Gy/fraction (α/β = 3), which appear to be safe dose levels [Citation9,Citation11,Citation28]. During RT however, the volume-receiving high doses increased up to 5 cm3.
In recent years, increasing attention has been directed at heart toxicity. Results from RTOG0617 indicate a correlation between heart dose and mortality [Citation4]. In this study, none of the patients presented a tumour adjacent to the heart. Hence, the current study showed no increase in mean dose or high-dose volumes to the heart.
In the current study, dose limits were set for all structures in the thorax. However, the effect of high doses to the different structures varies considerably, from chest pain to life-threatening toxicity in case of the oesophagus or heart being exposed to high doses. Therefore, the limit for the maximum allowable dose during RT should take this into account and, e.g., allow the dose to the chest wall to increase above the limit set in the protocol, whereas doses increasing above the limit, e.g., oesophagus and heart should trigger ART.
The current study is based on the heterogeneous dose escalation of the NARLAL2 trail. This provides a higher degree of freedom to increase target mean doses while allowing better OAR sparing than a homogeneous dose escalation would. For a homogeneous dose distribution of 74 Gy, the constraint violations would probably decrease in maximum dose but might increase in volume compared to the results of this study. Further, this study does not incorporate the fact that an anatomical change process such as tumour shrinkage would likely continue throughout the treatment course [Citation14,Citation25]. Neither does it account for anatomical changes that occur after fraction 10(−14), where the sCT was acquired to allow time for adaption. If ART is performed at this stage, at least part of the consequences of tumour shrinkage can be alleviated. However, if ART is not performed, chances are high that OAR constraint violations will be more pronounced in reality than observed in this study.
Previous studies reporting the effectiveness of ART are mainly focused on target coverage and only for treatment of lung cancer to standard doses [Citation13–15]. In the study population investigated here, target under-dosage occurs in 15% of the patients. This percentage is similar to what is observed for a large cohort treated with homogenous standard doses [Citation15]. They report that under-dosage is mainly caused by changes in atelectasis, tumour deformation and differential motion of tumour and lymph nodes. In this study, the primary cause of target under-dosage was differential motion of tumour and lymph nodes. We observed no under-dosage due to shrinkage, which coincides well with reports that tumour shrinkage can increase the tumour dose slightly [Citation31].
In conclusion, anatomical changes during radiotherapy compromised the otherwise strict OAR constraints of the NARLAL2 dose escalation trial in about half of the study population. Tumour shrinkage was identified as the main cause and we suggest frequent monitoring and the use of ART to avoid OAR toxicities. In the NARLAL2 trial, ART is mandatory for all participating centres.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15:23–34.
- Belderbos JS, Heemsbergen WD, De Jaeger K, et al. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:126–134.
- Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–333.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199.
- Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042–1044.
- Faivre-Finn C. Dose escalation in lung cancer: have we gone full circle? Lancet Oncol. 2015;16:125–127.
- Chen C, Uyterlinde W, Sonke JJ, et al. Severe late esophagus toxicity in NSCLC patients treated with IMRT and concurrent chemotherapy. Radiother Oncol. 2013;108:337–341.
- Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394.
- Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343–4348.
- Miller KL, Shafman TD, Anscher MS, et al. Bronchial stenosis: an underreported complication of high-dose external beam radiotherapy for lung cancer? Int J Radiat Oncol Biol Phys. 2005;61:64–69.
- Evans JD, Gomez DR, Amini A, et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother Oncol. 2013;106:327–332.
- Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62.
- Møller DS, Khalil AA, Knap MM, et al. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother Oncol. 2014;110:517–522.
- Kwint M, Conijn S, Schaake E, et al. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol. 2014;113:392–397.
- Møller DS, Holt MI, Alber M, et al. Adaptive radiotherapy for advanced lung cancer ensures target coverage and decreases lung dose. Radiother Oncol. 2016;121:32–38.
- Møller DS, Nielsen TB, Brink C, et al. Heterogeneous FDG-guided dose-escalation for locally advanced NSCLC (the NARLAL2 trial): design and early dosimetric results of a randomized, multi-centre phase-III study. Radiother Oncol. Forthcoming. [cited 2017 Jul 5]. DOI:10.1016/j.radonc.2017.06.022
- Wolthaus JW, Schneider C, Sonke JJ, et al. Mid-ventilation CT scan construction from four-dimensional respiration-correlated CT scans for radiotherapy planning of lung cancer patients. Int J Radiat Oncol Biol Phys. 2006;65:1560–1571.
- Underberg RW, Lagerwaard FJ, Slotman BJ, et al. Use of maximum intensity projections (MIP) for target volume generation in 4DCT scans for lung cancer. Int J Radiat Oncol Biol Phys. 2005;63:253–260.
- Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81:1442–1457.
- Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18.
- Lodge MA, Chaudhry MA, Wahl RL. Noise considerations for PET quantification using maximum and peak standardized uptake value. J Nucl Med. 2012;53:1041–1047.
- Rønde H, Hoffmann L. Validation of Varian’s AAA algorithm with focus on lung treatments. Acta Oncol. 2009;48:209–215.
- Hoffmann L, Holt MI, Knap MM, et al. Anatomical landmarks accurately determine interfractional lymph node shifts during radiotherapy of lung cancer patients. Radiother Oncol. 2015;116:64–69.
- Hansen O, Knap MM, Khalil A, et al. A randomized phase II trial of concurrent chemoradiation with two doses of radiotherapy, 60 Gy and 66 Gy, concomitant with a fixed dose of oral vinorelbine in locally advanced NSCLC. Radiother Oncol. 2017;123:276–281.
- Knap MM, Hoffmann L, Nordsmark M, et al. Daily cone-beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol. 2010;49:1077–1084.
- Schaake EE, Rossi MMG, Buikhuisen WA, et al. Differential motion between mediastinal lymph nodes and primary tumor in radically irradiated lung cancer patients. Int J Radiat Oncol Biol Phys. 2014;90:959–966.
- van Elmpt W, De Ruysscher D, van der Salm A, et al. The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol. 2012;104:67–71.
- Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463.
- Kimsey F, McKay J, Gefter J, et al. Dose-response model for chest wall tolerance of stereotactic body radiation therapy. Semin Radiat Oncol. 2016;26:129–134.
- Uyterlinde W, Chen C, Belderbos J, et al. Fractures of thoracic vertebrae in patients with locally advanced non-small cell lung carcinoma treated with intensity modulated radiotherapy. Radiother Oncol. 2016;118:437–441.
- Sibolt P, Ottosson W, Sjöström D, et al. Adaptation requirements due to anatomical changes in free-breathing and deep-inspiration breath-hold for standard and dose-escalated radiotherapy of lung cancer patients. Acta Oncol. 2015;54:1453–1460.
