Introduction
In 2016, anal cancer (AC) incidence was estimated to be 8080 which represented 0.5% of all cancer cases [Citation1]. Despite advancing technology, the overall treatment paradigm has not changed since Nigro et al. [Citation2] first published their results of chemoradiotherapy in the early 1970s. This regimen is very efficacious but is not without side effects. Bone marrow (BM) suppression is a significant risk with rates of acute Grade 3–4 hematologic toxicity (G3 + HT) ranging from 26% to 61% [Citation3–5]. This toxicity is thought in large part to be secondary to the volume of pelvic bone marrow receiving radiation. These treatment related cytopenias can lead to unplanned treatment breaks, chemotherapy dose reductions, transfusions, and infections.
To help lower this risk, dosimetric parameters to reduce hematologic toxicity (HT) have been investigated. Mell et al. first reported on V10 and V20 of pelvic bone marrow (PBM) being associated with acute leukopenia and neutropenia in patients with AC and subsequent studies have shown similar results [Citation6–12]. Recent studies suggest that BM, much like the liver, is a parallel organ and the volume spared a threshold dose may better predict acute HT [Citation10,Citation13]. In this report, we hypothesize that the PET-defined active pelvic bone marrow (APBM) volume spared a pre-specified dose will better predict the rate of G3 + HT compared to conventional dosimetric parameters. We defined the APBM as the PBM volume with an SUV greater than or equal to the total body mean SUV.
Material and methods
We retrospectively reviewed 18 patients with the diagnosis of AC who received definitive radiotherapy with concurrent mitomycin (MMC)/fluoropyrimidine based chemotherapy at a single institution between 2010 and 2015 under guidance from the institutional review board. PET CT imaging was obtained prior to therapy and was fused in MIMvista (Mimvista, Cleveland, OH, USA) to the simulation CT for target delineation. Radiotherapy was delivered using IMRT, volumetric arc therapy (VMAT), or helical tomotherapy. Target volumes, dosing, and dose constraints were defined by RTOG 0529 [Citation14].
The PBM was contoured similarly to Mell et al. [Citation12] using bone windows on the simulation CT. As previously stated, the APBM was defined as the region of PBM with an SUV greater than or equal to the total body mean SUV consistent with previous works () [Citation7]. The total body mean SUV was derived in MIMvista using the pretreatment PET CT fused with the simulation CT.
Figure 1. Representative volumes for pelvic bone marrow (pink) and active pelvic bone marrow (orange) contoured on axial (A) as well as coronal (B) CT slices.
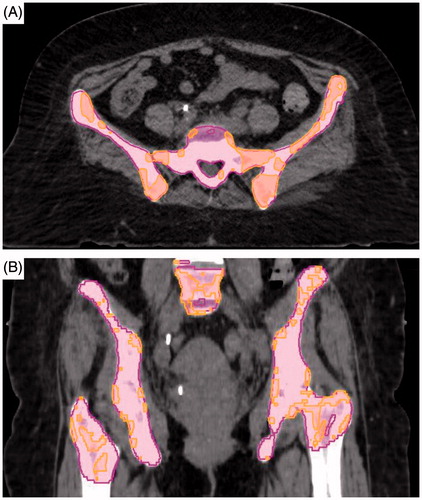
For each patient, we evaluated conventional and volume based dosimetry metrics. HT was defined using the common terminology criteria for adverse events (CTCAE), version 4.03 [Citation15]. G3 + HT was defined as any G3 + toxicity for hemoglobin (Hb), absolute neutrophil count (ANC), platelets (Plt), or white blood cell (WBC) based on weekly labs concurrent with and one month post treatment. Receiver operator characteristic (ROC) curves were performed comparing the predefined dosimetric continuous variables against the binary variable of G3 + HT. Fisher’s exact test was then used to evaluate significant variables using a two-sided p value. Statistical analysis was performed using MedCalc® version 15.11.4.
Results
Patient characteristics are described in . The median total dose of radiation to the primary PTV was 54.0 Gy (39.6–60). The mean volumes for PBM and APBM were 1532 cc (1189–2028) and 941 cc (291–1486), respectively. The mean doses to the PBM and APBM were 30 Gy (20–37) and 29 Gy (17–38), respectively. The primary toxicity endpoint was G3 + HT which occurred in 44% (8/18) of the cohort.
Table 1. Characteristics (n = 18).
The results of the ROC curve analysis are displayed in . The V10, V20, V40, and mean dose did not significantly predict for G3 + HT for APBM or PBM. The baseline APBM volume of 954 cc (AUC 0.788, p = .017) was shown to be a significant predictor of G3 + HT; however, the volume of PBM were nonsignificant. An APBM volumetric sparing goal of 216 cc was significant at a threshold dose of 20 Gy (AUC 0.788, p = .015). APBM volume of 564 cc spared at a threshold dose of 40 Gy was also significant (AUC 0.788, p = .016). The volume of PBM spared at threshold doses of 10 Gy, 20 Gy, 40 Gy and APBM spared at 10 Gy were nonsignificant. In patients with ≤64 cc of APBM spared 40 Gy, 86% (6/7) developed G3 + HT compared to 18% (2/11) of patients with >564 cc spared 40 Gy (p = .0128). In patients with ≤216 cc of APBM spared 20 Gy, 70% (7/10) developed G3 + HT compared to 13% (1/8) of patients with >216 cc spared 20 Gy (p = .0248).
Table 2. ROC analysis for grade 3 + hematologic toxicity.
Discussion
This study is the first to report that the volume of PET-defined APBM spared a threshold dose can predict acute G3 + HT in patients treated with definitive chemoradiation for AC confirming our initial hypothesis. The rate of acute G3 + HT in our cohort appears to be similar to those presented previously in the literature.
Mell et al. [Citation12] was first to publish an analysis correlating WBC/ANC nadirs with radiation dose to PBM in AC. The authors were also able to confirm the hypothesis that a large volume of low-dose radiation to the PBM is associated with acute HT. IMRT has been attempted to reduce this low-dose radiation volume using the PBM as a surrogate for BM but this can be challenging due to competing goals of reducing bowel dose and maintaining target coverage. Therefore, identifying smaller volumes of active BM within the bony compartment is of interest. Using FDG-PET, several investigators have evaluated whether metabolically active regions of BM may better represent the BM compartment at risk. Rose et al. first evaluated using the APBM as a predictor for HT which was validated by Franco et al. [Citation7,Citation9]. These studies showed a significant correlation using linear modeling at various APBM values for ANC and WBC nadirs but not Plt or Hb.
Active pelvic bone marrow use has been validated in cervical cancer in the recently published INTERTECC-2 trial [Citation16]. This study tested the hypothesis that BM sparing IMRT with concurrent cisplatin could reduce rates of G3 + neutropenia or significant gastrointestinal toxicity. A preplanned subgroup analysis evaluating PET CT image-guided IMRT to spare functional BM showed significantly lower rates of G3 + neutropenia (8.6% vs 27.1%, p = .035) in patients who received APBM sparing IMRT. The current NRG-GY006 phase II study in cervical or vaginal cancer evaluating definitive chemoradiation with or without triapine is also currently using similar dose constraints to the INTERTECC-2 (NCT02466971).
Although constraints have varied in regard to predicting HT, there appears to be a consistent trend that a large volume of low dose radiation to the PBM is strongly correlated with the development of G3 + HT. This property coincides with the PBM being a parallel organ where its functional capacity depends on the number of functional subunits (FSU) [Citation13]. In synthetic parallel structures, such as the liver and BM, a threshold volume spared a specific dose is important in predicting toxicity. Schefter et al. [Citation17] demonstrated this in the liver describing low rates of hepatotoxicity with ≥700 cc of normal liver receiving <15 Gy. A recent study by Lee et al. [Citation10] tested this hypothesis in AC patients receiving concurrent chemoradiation and showed a threshold volume of 750 cc of PBM spared 30 Gy was able to predict G3 + leukopenia and neutropenia. Patients with ≥750 cc spared 30 Gy had 5% rates of G3 + leukopenia and neutropenia vs 54% for those not meeting this constraint (p < .01). Our study did not replicate this threshold volume but we did support the conclusion that the volume of active bone marrow spared may be an important constraint for future studies. Potential reasons for our lack of significance in looking at the volume of PBM spared may be related to our small cohort size and our larger PBM volume (L4 was included which is more commonly performed in cervical cancer). However, since previous studies have shown the APBM to be the most sensitive region of the bone marrow compartment, the absolute volume of APBM volume spared is rationale constraint.
Conclusions
Despite the use of IMRT, there has been minimal reduction in the rate of G3 + HT. With improved radiation techniques and understanding of the relationship APBM has with the development of hematologic toxicity, the morbidity associated with AC treatment may be further reduced. In this report, we demonstrate a practical method of measuring APBM spared as well as its ability to predict G3 + HT. Patients with low baseline APBM appear to be at a higher risk of HT and this suggests that these patients may have insufficient baseline hematologic reserves. The use of APBM as a volume sparing constraint shows promise and warrants further evaluation in additional cohorts.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cancer of the anus, anal canal, and anorectum: cancer stat facts. 2017; [cited 2017 Feb 10]. Available from: https://seer.cancer.gov/statfacts/html/anus.html.
- Nigro ND, Vaitkevicius VK, Considine B. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–356.
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921.
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539.
- James RD, Glynne-Jones R, Meadows HM, et al., Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013:14;516–524.
- Cheng JCH, Bazan JG, Wu JK, et al. Lumbosacral spine and marrow cavity modeling of acute hematologic toxicity in patients treated with intensity modulated radiation therapy for squamous cell carcinoma of the anal canal. Pract Radiat Oncol. 2014;4:198–206.
- Rose B, Jee KW, Niemierko A, et al. Irradiation of FDG-PET defined-active bone marrow subregions and acute hematologic toxicity in anal cancer patients undergoing chemoradiation. Int J Radiat Oncol. 2016;94:747–754.
- Franco P, Ragona R, Arcadipane F, et al. Dosimetric predictors of acute hematologic toxicity during concurrent intensity-modulated radiotherapy and chemotherapy for anal cancer. Clin Transl Oncol. 2016;19:67–75.
- Franco P, Arcadipane F, Ragona R, et al. Dose to specific subregions of pelvic bone marrow defined with FDG-PET as a predictor of hematologic nadirs during concomitant chemoradiation in anal cancer patients. Med Oncol. 2016;33:72.
- Lee AY, Golden DW, Bazan JG, et al. Hematologic nadirs during chemoradiation for anal cancer: temporal characterization and dosimetric predictors. Int J Radiat Oncol. 2016;97:306–312.
- Bazan J, Luxton GG, Mok EC, et al. Normal tissue complication probability modeling of acute hematologic toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2012;84:700–706.
- Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1431–1437.
- Fry RJM, Hall EJ. Radiobiology for the radiologist. Radiat Res. 1995;141:327–356.
- Kachnic L, Winter K, Myerson R, et al. RTOG 0529: a phase II evaluation of dose-painted IMRT in combination with 5-fluorouracil and mitomycin-C for reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol. 2009;86:27–33.
- Protocol Development | CTEP. 2017; [cited 2017 Feb 10]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- Mell L, Sirak K, Wei IL, etet al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys. 2017;97:536–545.
- Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378.
