Abstract
Background: Hyponatremia has recently been associated with subsequent cancer risk. This population-based nationwide study assessed whether the diagnosis of hyponatremia can predict a cancer diagnosis within most common cancers.
Material and methods: Using Danish medical registries, we identified 16,220 patients with a first-time diagnosis of hyponatremia, without a cancer diagnosis, from January 2006 through November 2013. We quantified the relative risk of a subsequent cancer diagnosis by standardized incidence ratios (SIRs), comparing observed cancer incidence among patients diagnosed with hyponatremia to that expected, based on national cancer incidence during that period.
Results: During 40,207 person-years of follow-up, we observed 1546 cancer diagnoses compared to 956 expected (SIR: 1.62; 95% confidence interval (CI), 1.54–1.70). The increase in risk of a cancer diagnosis following a hyponatremia diagnosis was most pronounced within 0–6 months of follow-up (SIR 4.16; 95% CI, 3.85–4.48) and in the younger age group; 0–29 years (SIR 8.71; 95% CI, 2.82–20.28), 30–49 years (SIR 3.16; 95% CI, 2.26–4.31), 50–69 years (SIR 2.29; 95% CI, 2.10–2.48) and 70 + years (SIR 1.35; 95% CI, 1.27–1.44). Within six months after a hyponatremia diagnosis, the SIRs increased 10-fold for cancers of the lung (SIR 17.14; 95% CI, 15.15–19.32), brain (SIR 13.52; 95% CI, 8.90–19.66) and liver (SIR 13.26; 95% CI, 7.57–21.53) and increased 5 to 10-fold for cancers of the pancreas (SIR 8.25; 95% CI, 5.72–11.53), esophagus (SIR 6.59; 95% CI, 3.15–12.12), kidney (SIR 6.36; 95% CI, 3.39–10.88), pharynx (SIR 6.15; 95% CI, 1.27–17.97) and non-Hodgkin lymphoma (SIR 6.10; 95% CI, 4.17–8.61). The rate increased across virtually all types of cancers, except melanoma and basal cell carcinomas.
Conclusions: A diagnosis of hyponatremia may be a marker of occult neoplasms, especially cancers of the lung, brain, liver, pancreas, esophagus, kidney, pharynx and non-Hodgkin lymphoma. Hyponatremia may aid in early detection of cancer.
Background
Hyponatremia (serum sodium <135 mmol/l) is a common condition occurring in 18 to 47% of hospitalized cancer patients [Citation1–3] and has been linked to a poorer prognosis in patients with small and non-small cell lung cancer [Citation4,Citation5], hepatocellular carcinoma [Citation6], metastatic renal cell carcinoma [Citation7], colorectal cancer [Citation3] and cancer overall [Citation1]. Sodium depletion secondary to malnutrition, gastrointestinal or renal losses and cancer treatments are common mechanisms of hyponatremia in patients with advanced cancers [Citation8]. Hyponatremia may also be caused by paraneoplastic syndromes of ectopic inappropriate antidiuretic hormone secretion (SIADH), a condition well described in small cell lung cancers [Citation9–11] and head and neck cancers, hematological cancers, primary brain tumors, gastrointestinal cancers, breast and prostate cancer and several other malignancies [Citation11–13]. Furthermore, hyponatremia may be linked to cancerous disease through inflammatory interleukin 6 mediated vasopressin release [Citation14,Citation15].
Whether hyponatremia may precede clinical evident tumors and be a presenting symptom of cancer is unclear [Citation8,Citation16]. Sporadic case reports of hyponatremia preceding cancer diagnosis have been published [Citation16,Citation17]. A recent larger study among residents of Copenhagen (capital of Denmark) undergoing serum sodium measurement at their general practitioner, revealed increased incidence within one year of pulmonary cancers and head and neck cancers in patients with hyponatremia compared to patients with normonatremia [Citation18].
The present study is a population-based nationwide study examining whether the diagnosis of hyponatremia can predict a cancer diagnosis within the 20 most common cancers and thereby help in early cancer detection.
Material and methods
This cohort study was conducted within the tax-supported Danish healthcare system, through individual-level linkage between registries, based on a 10-digit unique civil personal registration number issued to all Danish citizens upon birth or immigration since 1968 [Citation19]. The Danish National Patient Registry (DNPR) contains information on all non-psychiatric hospitalizations in Denmark since 1977 and outpatient and emergency department visits since 1995 [Citation20].
We identified inpatients of all ages registered in the DNPR with a first-time diagnosis of hyponatremia from January 2006 through November 2013 (see Appendix A, supplemental online material for International Classification of Diseases, 10th Revision [ICD-10] codes). We identified incident cancers in this cohort, through individual level linkage to the Danish Cancer Registry (DCR) [Citation21]. Since 1943 all incident malignant neoplasms have been reported to the DCR. Electronic reporting through the DNPR and a dedicated platform for general practitioners and outpatient specialists was implemented in 2004–2005. Reporting is mandatory and an automated cancer logic algorithm determines whether the patient’s cancer disease is previously recorded in the Cancer Registry, ensuring that only incident cancers are registered [Citation22]. Patients diagnosed with solid tumors, hematologic or disseminated cancers before the hospital admission with a hyponatremia diagnosis were excluded (see Appendix A, supplemental online material for ICD-10 cancer diagnoses).
We followed each patient from the date of the first hyponatremia diagnosis until the date of first diagnosis of any type of cancer, death, emigration, or 30 November 2013, whichever came first. We reported the number and proportion of patients according to gender, age at hyponatremia diagnosis and calendar period. As a measure of relative risk, we estimated the standardized incidence ratio (SIR) with 95% confidence intervals (CIs), comparing cancer incidence observed among patients diagnosed with hyponatremia with that expected in the general Danish population, based on national cancer incidence rates by gender and single year of age and calendar year. We computed SIRs for 0– < 6 months of follow-up, 6– < 12 months of follow-up, >12 months of follow-up and for the total duration of follow-up in all cancers combined and in subcategories based on cancer site. Cancers diagnosed within the first six months were considered to represent occult cancers. SIRs were reported for the 20 cancer sites contributing most cases during the study period. We repeated the analysis stratified by age, gender and calendar year of hyponatremia diagnosis.
To examine whether our results were biased by suspected cancers not yet reported to the DCR at the time of hyponatremia diagnosis, we performed a sensitivity analysis also excluding patients with a cancer diagnosis or a diagnosis of ‘observation for suspected malignant neoplasm’ (see Appendix A, supplemental online material for ICD-10 diagnosis) registered in the DNPR within one year before the hyponatremia diagnosis.
All statistical analyses were performed using SAS V 9.4. The study was approved by the Danish Data Protection Agency (Central Denmark record number 1-16-02-1-08).
Results
We identified 16,220 patients with a first-time diagnosis of hyponatremia during 2006–2013 and followed them for a total of 40,207 person-years (median follow-up time 2 years [interquartile range 0.6 to 4]). Among these patients 10,669 (65.8%) were women. Approximately 61% (n = 9834) were aged 70 years or older, 5.5% (n = 897) were 30 to 49 years of age and 2.7% (n = 430) were younger than 30 years of age. Median age was 74.8 years (interquartile range 63.0 to 83.9).
Standardized incidence ratios for cancer
Overall, 1546 (9.5%) cancers occurred among the 16,220 patients diagnosed with hyponatremia during the study period. The expected number of cancers in the general population standardized on gender, age and calendar year was 956, yielding an overall SIR of 1.62 (95% CI, 1.54–1.70) (). Hyponatremic patients regardless of gender and age were at increased risk of cancer, with SIRs being higher in men (SIR 1.86; 95% CI, 1.71–2.01) than in women (SIR 1.50; 95% CI, 1.41–1.60) and higher in the youngest age group of 0–29 years (SIR 8.71; 95% CI, 2.82–20.28) compared to patients aged 30–49 years (SIR 3.16; 95% CI, 2.26–4.31), 50–69 years (SIR 2.29; 95% CI, 2.10–2.48) and ≥70 years (SIR 1.35; 95% CI, 1.27–1.44), respectively.
Table 1 SIRs for cancer in patients diagnosed with hyponatremia in Denmark from 2006–2013.
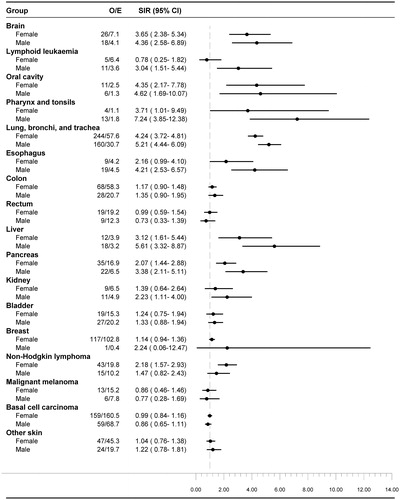
In total, 691 of the 1546 cancers were diagnosed within six months after the hyponatremia diagnosis. The expected number of cancers in the general population during the same period was 166, corresponding to a SIR of 4.16 (95% CI, 3.85–4.48) (). Within this timeframe, the rate was increased across virtually all types of cancers, except malignant melanoma and basal cell carcinomas ( and Appendix B, supplemental online material for SIRs by cancer type and follow-up period). The increase in risk was most pronounced in cancers of the lung (SIR 17.14; 95% CI, 15.15–19.32), liver (SIR 13.26; 95% CI, 7.57–21.53) and brain (SIR 13.51; 95% CI, 8.90–19.66). For most cancer types, SIR remained elevated for an observation period longer than six months. Even after one year, a strong association persisted between hyponatremia and cancers of the oral cavity, pharynx and tonsils, lung, esophagus and liver ( and Appendix B, supplemental online material). In most types of cancer, male patients were at higher risk that female patients. The difference was particularly evident in cancers of lung, pharynx, esophagus, liver and pancreas ().
Figure 1. SIRs by follow-up period for the ten most frequent cancers in patients with hyponatremia. CI: confidence interval; E: expected; O: observed, SIR: standardized incidence ratios
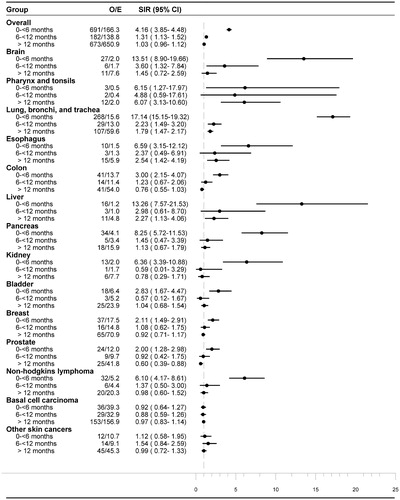
Figure 2. SIRs by type of cancer and gender in patients with hyponatremia. CI: confidence interval; E: expected; O: observed, SIR: standardized incidence ratios
A sensitivity analysis excluding patients ever registered with a cancer diagnosis in the DNPR or an ‘observation for suspected malignant neoplasm’ diagnosis within one year before the hyponatremia diagnosis, resulted in a slightly decreased SIR 1.55 (95%CI, 1.47–1.63) (compared with SIR 1.62 (95%CI, 1.54–1.70) in the main analysis), but had modest impact on the remaining results (Appendix C and D, supplemental online material).
Discussion
This nationwide population-based study demonstrated a markedly increased risk of being diagnosed with cancer during the first six months following a hyponatremia diagnosis. The risk was particularly increased in the younger age groups. A more than 10-fold standardized incidence ratio was seen for cancers of the lung, brain and liver and a five to 10 fold increased ratio for pancreas, esophagus, kidney and pharynx and non-Hodgkin lymphoma. As the elevated risk persisted beyond the first year of follow up in several cancer types, it is unlikely that these observations were caused entirely by diagnostic bias. These cancers are all characterized with few early warning symptoms; resulting in an advanced and often incurable stage at primary diagnosis. The identification of hyponatremia as a potential early predictor of specific cancers should be further analyzed in larger and prospective studies, including screening programs. The implication is hyponatremia may aid in early detection of cancer.
The relevance of hyponatremia as marker of cancer has only been sparsely investigated. We were able to examine the risk of the 20 most frequent cancers, at different time intervals after a hyponatremia diagnosis and in different age-groups, thereby making a substantial contribution to current knowledge. The observed increased risk of lung cancer, cancers of the oral cavity, pharynx and tonsils concur well with the findings of Selmer and colleagues [Citation18] and the well-described proneness of these cancers to induce SIADH [Citation9–13]. The study by Selmer et al. [Citation18] did not distinctively investigate the risk of cancer of for example the brain or liver. Nevertheless, reports of SIADH exist for most of the cancers in which we observed an increased risk [Citation11,Citation13]. Not only the proneness to induce SIADH, but also different predisposition of each cancer type to stimulate an inflammatory response and thereby hyponatremia, could explain the difference in risk observed [Citation14,Citation15]. Ultimately, it will be important to determine whether cancer screening in patients with hyponatremia will result in earlier detection of cancers assessed by lower cancer stage and decreased mortality, as well as decreased hospital resource utilization [Citation1,Citation23].
The strengths of our study include its nationwide population-based design within the setting of a uniform tax-supported healthcare system with complete follow-up of all patients [Citation19]. However, some limitations exist. First, the validity of our results depends on the quality of diagnoses recorded in the registries. The data quality of all major cancer diagnoses recorded in the DCR is high [Citation21]. Also, the predictive value of hyponatremia diagnoses recorded in the DNPR is very high [Citation24]. Second, transient hyponatremia caused by abnormalities in water homeostasis is very common and is occurring in up to 20% of hospitalized patients and therefore probably not coded in the hospital registry. In fact, less than 2% of inpatients who experience a hyponatremia episode during their hospital stay receive a formal registry diagnosis [Citation24]; such misclassification would most likely underestimate the true relative risk, but it also implies that our results may not apply to transient hyponatremic episodes during hospital stay, but to patients with a true hyponatremia diagnosis. Patients who receive a hyponatremia diagnosis tend to have more severe hyponatremia, be older and have lower morbidity burden than patients with hyponatremia who do not receive a diagnosis [Citation24]. In concordance, Selmer et al. [Citation18] found higher risk of being diagnosed with cancer in patients with serum sodium <125 mmol/l compared to less severe hyponatremia. Considering the magnitude of the SIRs estimates both overall and for the specific cancer types, bias due to prevalent exposure in the general population is likely negligible [Citation25].
For most cancers, the increase in risk was most pronounced within six months after the hyponatremia diagnosis. With reference to the discussion above, regarding the potential bias entailed by the underreporting of hyponatremia, it is likely that our study primarily included patients with moderate to severe hyponatremia for which there was no obvious or recognized cause and that hyponatremia in itself trigged further diagnostic work-up leading to the cancer diagnosis. This would certainly be in line with current recommendations [Citation26]. Pointing in that direction, we found that a smaller proportion of cancers were diagnosed between six and 12 months after the hyponatremia diagnosis. Also, the probability of detecting cancers may be increased in hospitalized patients. Yet, for cancers of the oral cavity, pharynx and tonsil, esophagus and liver we observed a more than two fold increased risk of cancer even after one year. Although not of the same magnitude, the risk also remained elevated in many other cancers, which suggest that surveillance bias cannot entirely explain the overall increased risk of a cancer diagnosis.
We conclude that hyponatremia may be a marker of prevalent occult cancer, especially cancers of the lung, brain, liver, pancreas, esophagus, kidney, pharynx and non-Hodgkin lymphoma, which merits attention when providing guidelines for cancer surveillance programs and the clinical work-up of patients with hyponatremia. Future investigations should seek to answer whether early diagnosis prompted by detection of hyponatremia can affect cancer survival.
IONC_A_1378430_Supplementary_Information.zip
Download Zip (23.5 KB)Disclosure statement
Jens Otto Lunde Jørgensen has received an unrestricted research grant from Otsuka Pharma Scandinavia AB; Louise Holland-Bill and Jens Otto Lunde Jørgensen have received lecture fees from Otsuka Pharma Scandinavia AB; Louise Holland-Bill, Christian Fynbo Christiansen, Dóra Körmendiné Farkas and Henrik Toft Sørensen are employees at the Department of Clinical Epidemiology, Aarhus University Hospital. The Department of Clinical Epidemiology, Aarhus University Hospital receives funding from companies in the form of research grants to (and administered by) Aarhus University.
Additional information
Funding
References
- Doshi SM, Shah P, Lei X, et al. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis. 2012;59:222–228.
- Holland-Bill L, Christiansen CF, Heide-Jorgensen U, et al. Hyponatremia and mortality risk: a Danish cohort study of 279 508 acutely hospitalized patients. Eur J Endocrinol. 2015;173:71–81.
- Choi JS, Bae EH, Ma SK, et al. Prognostic impact of hyponatraemia in patients with colorectal cancer. Colorectal Dis. 2015;17:409–416.
- Tiseo M, Buti S, Boni L, et al. Prognostic role of hyponatremia in 564 small cell lung cancer patients treated with topotecan. Lung Cancer. 2014;86:91–95.
- Kobayashi N, Usui S, Yamaoka M, et al. The influence of serum sodium concentration on prognosis in resected non-small cell lung cancer. Thorac Cardiovasc Surg. 2014;62:338–343.
- Huo TI, Lin HC, Hsia CY, et al. The MELD-Na is an independent short- and long-term prognostic predictor for hepatocellular carcinoma: a prospective survey. Dig Liver Dis. 2008;40:882–889.
- Schutz FA, Xie W, Donskov F, et al. The impact of low serum sodium on treatment outcome of targeted therapy in metastatic renal cell carcinoma: results from the international metastatic renal cell cancer database consortium. Eur Urol. 2014;65:723–730.
- Onitilo AA, Kio E, Doi SA. Tumor-related hyponatremia. Clin Med Res. 2007;5:228–237.
- List AF, Hainsworth JD, Davis BW, et al. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4:1191–1198.
- Bondy PK, Gilby ED. Endocrine function in small cell undifferentiated carcinoma of the lung. Cancer. 1982;50:2147–2153.
- Berghmans T, Paesmans M, Body JJ. A prospective study on hyponatraemia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer. 2000;8:192–197.
- Talmi YP, Hoffman HT, McCabe BF. Syndrome of inappropriate secretion of arginine vasopressin in patients with cancer of the head and neck. Ann Otol Rhinol Laryngol. 1992;101:946–949.
- Sorensen JB, Andersen MK, Hansen HH. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in malignant disease. J Intern Med. 1995;238:97–110.
- Mastorakos G, Weber JS, Magiakou MA, et al. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. 1994;79:934–939.
- Park SJ, Shin JI. Inflammation and hyponatremia: an underrecognized condition? Korean J Pediatr. 2013;56:519–522.
- Sejling AS, Thorsteinsson AL, Pedersen-Bjergaard U, et al. Recovery from SIADH-associated osteoporosis: a case report. J Clin Endocrinol Metab. 2014;99:3527–3530.
- Kamoi K, Kurokawa I, Kasai H, et al. Asymptomatic hyponatremia due to inappropriate secretion of antidiuretic hormone as the first sign of a small cell lung cancer in an elderly man. Intern Med. 1998;37:950–954.
- Selmer C, Madsen JC, Torp-Pedersen C, et al. Hyponatremia, all-cause mortality and risk of cancer diagnoses in the primary care setting: a large population study. Eur J Intern Med. 2016;36:36–43.
- Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- Schmidt M, Schmidt S, Sandegaard J, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;17:449–490.
- Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry-history, content, quality and use. Dan Med Bull. 1997;44:535–539.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45.
- Gisby M, Lundberg J, Ländin M, et al. The burden of illness in patients with hyponatremia in Sweden: a population-based registry study. Int J Clin Pract. 2016;70:319–329.
- Holland-Bill L, Christiansen CF, Ulrichsen SP, et al. Validity of the international classification of diseases, 10th revision discharge diagnosis codes for hyponatremia in the National Registry of Patients. BMJ Open. 2014;4:e004956.
- Jones ME, Swerdlow AJ. Bias in the standardized mortality ratio when using general population rates to estimate expected number of deaths. Am J Epidemiol. 1998;148:1012–1017.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126:S1–S42.
