Abstract
Background: Fractionated therapy with 177Lu-DOTATATE has been reported to be an effective treatment for patients with metastasized neuroendocrine tumors. To optimize the treatment, absorbed doses to risk organs are calculated for the individual patient. For each organ, absorbed dose due to activity in the organ itself (self-dose) and that originating from other organs (cross-dose) are calculated from serial measurements to obtain the activity distribution following treatment. The main aim of the present work were to calculate the cross-dose contribution to the total absorbed kidney dose.
Methods: Five hundred patients with neuroendocrine tumors undergoing therapy with 177Lu-DOTATATE were included. Scintigraphic planar whole body images and single photon emission computed tomography/computed tomography (SPECT/CT) over the abdomen were acquired at 1, 4 and 7 days after treatment. Kidney self-dose was calculated based on radioactivity distribution obtained from SPECT/CT. Cross-dose to kidneys was estimated using organ-based analysis of planar whole body images and cross-fire dose factors from Olinda/EXM 1.1.
Results: Cross-dose to kidneys in the majority of patients were less than 2% and almost all cross-doses were less than 10%. Cross-dose exceeded 10% only in rare cases of patients with high tumor burden and low absorbed doses to kidneys.
Conclusions: The absorbed dose from 177Lu-octreotate to solid organs due to cross-fire is generally low and can usually be neglected.
Introduction
During the last decade peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTA-D-Phe1-Tyr3-octreotate (177Lu-DOTATATE) has evolved as a treatment option for patients with disseminated somatostatin receptor positive neuroendocrine tumors [Citation1–5]. Similarly to the situation in external beam radiation therapy, it is assumed that PRRT should aim to deliver as high absorbed dose as possible to the tumors, while at the same time the absorbed doses to normal organs, especially those at risk such as kidneys and bone marrow, should be kept within the safety margins [Citation1,Citation6–10]. Supporting this, it has been shown that the therapeutic effect (tumor shrinkage) of PRRT on pancreatic neuroendocrine tumors correlates with the absorbed dose to the tumor [Citation11].
The safety limits for the organs at risk at PRRT have been adopted from dosimetry applied in external beam radiation therapy where an absorbed dose of 23 Gyto the kidneys results in a 5% risk over 5 years (TD5/5) to develop radiation nephritis [Citation12]. This has earlier been determined to correspond to a biological effective dose (BED) of 38 Gy [Citation13]. It was also pointed out that the uncertainty of this value, due to variance in α/β values, would be approximately 20% or 8 Gy [Citation13]. The conditions during PRRT are, however, different. Because of the findings of a heterogeneous activity distribution in the kidneys at a microscopic level, 29 Gy has been proposed as the maximum absorbed dose to the kidneys [Citation14]. Moreover, because of the low dose rate in PRRT a maximum BED of 45 Gy for the kidneys was suggested for PRRT using 90Y-DOTATOC [Citation15].
The current standard in 177Lu-DOTATATE therapy is generally to administer four 7.4 GBq cycles (29.6 GBq in total) which is considered safe for the organs at risk. However, since only 1/74 patients in a recent study [Citation16] showed significant renal toxicity ( ≥ grade 3) after receiving 14.8 to 37.8 GBq of 177Lu-DOTATATE, the administered activity to the individual patient could most probably be increased.
In PRRT using 177Lu-DOTATATE, calculation of the absorbed dose is based on the activity distribution and kinetics over time for the relevant organs and tissues, which generally is obtained by repeated gamma camera measurements over time. This is followed by interpolations between the measurements and extrapolations before the first and after the last measurement points. The extrapolations should be as small as possible and include less than 20% of the decays in the volume [Citation17].
Since almost 80% of the energy from the 177Lu decay results from emission of short range beta particles, the absorbed dose for the kidneys originates mainly from the organs themselves (self-dose). In a simulation study [Citation18] it was stated that only a small percentage of the absorbed dose originated from the cross-dose from the surrounding organs and that the self-dose alone should therefore yield an accurate estimate of the absorbed dose. As previously reported [Citation19–21], the self-dose can be calculated by multiplying the activity concentration for an organ/volume at the given time point by an almost size independent activity concentration dose factor.
The main aims of this work were to calculate the contribution of the cross dose from other organs to the absorbed dose to the kidneys. Secondary aims were to calculate the BED to the kidney and to investigate the number of treatment cycles that may be performed taking into account the limits of absorbed dose and BED to kidneys or absorbed dose to bone marrow.
Material and methods
Patients
Five hundred patients (231 female and 269 male) with metastatic somatostatin receptor-expressing neuroendocrine tumors treated with 177Lu-DOTATATE were included and all met previously described inclusion criteria [Citation21]. Median age was 62.4 years (Range 18–84, mean 60.6). Forty-nine per cent of patients suffered from metastasized neuroendocrine tumors (NETs) of the small intestine and 26% from pancreaticoduodenal NETs. Baseline Chromogranin A was recorded in 125 patients and was elevated in 111 of them (89%). 5-HIAA was recorded for 72 patients, 69 of them had elevated levels. Out of 117 patients with initially increased tumor markers (5-HIAA, tumor hormones and/or chromogranin A) and sufficient follow-up, 65 had a documented decrease during and after therapy by more than 50% of the level prior to start of PRRT. Requirement for treatment were a S-creatinine <110 μmol or, if higher, GFR (cystatin-C) should be >50ml/min/1.73m2. Only patients with two kidneys were included in the analysis and thus all 500 patients were included in the analysis.
177LuCl3 was purchased from IDB Radiopharmacy bv, Baarle-Nassau, The Netherlands and DOTATATE was a generous gift from Erasmus Medical Centre, Rotterdam, The Netherlands.
Since September 2010 all patients were included into a prospective study (EudraCT no. 2009-012260-14) approved by the Regional Ethical Review Board in Uppsala. Before that from 2005 the patients were admitted on a single-patient basis for compassionate use with individual permission of the Swedish Medical Products Agency. All patients gave their written informed consent before inclusion.
Image acquisition
All 500 patients underwent SPECT/CT of the abdomen and whole-body scintigraphy (anterior and posterior planar acquisitions) 1, 4 and 7 days after administration of the first therapeutic cycle of 7.4 GBq 177Lu-DOTATATE. In this study the absorbed dose to later cycles were assumed to be the same as in the first cycle. This is a valid assumption on a group but not for individual patients where (as described earlier) the effective half-life from the first therapy was used together with one measurement point [Citation22]. The first 69 patients, underwent imaging on a Hawkeye Millennium VG (GE Healthcare) dual-head camera equipped with 5/8" NaI(Tl) crystals and MEGP (medium-energy general-purpose) collimators. A 20% energy window around the two dominant γ-ray energies of 177Lu, 113.0 and 208.4 keV was applied. SPECT/CT, applying 60 frames with a 60 second exposure time per frame (30 min SPECT acquisition time) was performed over the upper abdomen including organs at risk (kidneys, liver and spleen). Whole-body scanning at a scan speed of 7 cm/min was performed after each SPECT/CT. Immediately after the day 1 scan, a transmission scan using a 57Co planar source was acquired using a 20% energy window around 122 keV (scan speed 30 cm/min) and was then repeated without the 57Co planar source to determine the 177Lu background in the 122 keV energy window. In the next 400 patients imaging was performed on an Infinia (International General Electric, General Electric Medical Systems, Haifa, Israel) dual-headed gamma camera with 3/8" NaI(Tl)-crystals equipped with MEGP collimators. A 20% energy window was placed around the dominant 208.4 keV gamma ray energy of 177Lu to make the measurements. SPECT/CT of the upper abdomen including organs at risk, applying 120 frames with a 30 second exposure time per frame (30 min acquisition time for SPECT) was acquired and whole-body scanning was performed in the same way as for the VG camera. In the last 31 patients imaging was performed on an Discovery 670 PRO (International General Electric, General Electric Medical Systems, Haifa, Israel) dual-headed gamma camera with 3/8" NaI(Tl)-crystals equipped with MEGP collimators with the same settings as for the Infinia. For reconstruction, the ordered subsets expectation maximization (OSEM) algorithm included in the Xeleris 3.0 workstation (International General Electric, General Electric Medical Systems, Haifa, Israel) was used with previously determined default settings (iterative reconstruction with eight subsets and four iterations followed by a Hann filtering with a cutoff of 0.85). The images were attenuation corrected with the concomitantly acquired CT-based attenuation map.
Absorbed dose calculation
All volumes of interest and regions of interest were defined using in-house developed software within the Hermes platform on a Hermes HNAC workstation with Gold 2.9 software (HERMES, Stockholm, Sweden).
In the SPECT images, small spherical volumes of interests (4 ml) were placed in both kidneys over the region of the highest activity as described previously [Citation21]. This method (with PVE correction) has been shown to give an absorbed dose estimation that is close to a whole organ measurement [Citation19] and it is much less time consuming to perform. Activity concentrations were determined for each time point and time-integrated activity concentration was calculated as the area under the curve of a single exponential fit (from injection start to infinity) to the time-activity concentration curve. Absorbed doses (self-dose) to both kidneys were calculated by multiplication of time-integrated organ activity concentration with the appropriate activity concentration dose factor.
To calculate the absorbed dose to a target organ from other organs (cross-dose) according to what Flemming described earlier [Citation23], regions of interest were drawn on attenuation corrected geometric mean images obtained by planar whole-body scans at day 1 to delineate kidney, liver, spleen, tumor and whole body. These regions of interest were subsequently transferred to the day 4 and day 7 images and the data from the kidney, liver, spleen and tumor were fitted to a mono-exponential function. Absorbed doses (cross-dose) to kidneys were calculated by multiplication of time-integrated organ activity with the appropriate dose factor.
Absorbed doses to bone marrow were calculated as described earlier [Citation20] from blood samples assuming the same concentration to assess the self-dose and whole-body scans as described above to estimate the cross-doses.
BED calculation
The surviving fraction (S) of target cells of an absorbed dose (D) can be described by the linear-quadratic (LQ) model shown by:
(1)
In EquationEquation (1)(1) the double-strand breaks introduced by a single ionizing event are described by the linear component αD while the quadratic component βD2 describes the double-strand breaks introduced by two separate ionizing events.
Using this model, an estimation of the BED of a therapeutic absorbed dose delivered over a continuous period of time (following injection of 177Lu-DOTATATE) can be described and cumulative BED over a series of such treatment cycles is given by [Citation15]:
(2)
where Di is the delivered absorbed dose for cycle i, t1/2rep is the repair half-life of the organ and t1/2eff is the effective half-life of 177Lu-DOTATATE in the organ.
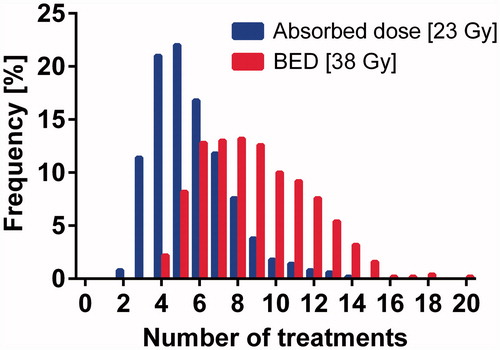
As reported in the literature [Citation24] for therapies with multiple fractions per day (in this case infinitely many) α/β for the kidney was set to 2.6 Gy and t1/2rep for the kidney was set to 2.8 h. The results of the BED calculations were also used, assuming the same kidney and bone marrow dose for a given patient on repeated treatment cycles, to determine the number of cycles that could be administered to each patient with a maximum of 23 Gy (absorbed dose) or 38 Gy (BED) to the kidneys [Citation13], both taking into account the limit of 2 Gy (absorbed dose) to the bone marrow as well. The percentage of patients who would reach the dose limit to the kidney or bone marrow was calculated.
Statistical methods
A Wilcoxon matched-pair signed rank test was used to compare data for the right and left kidney and a p < .05 was considered significant.
Results
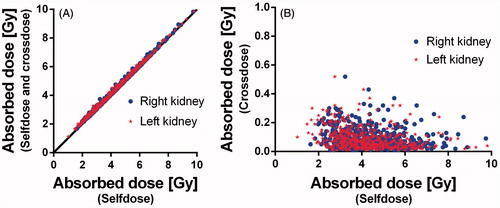
shows the relation between the self-dose and the total absorbed dose (self-dose and cross dose) ((A)) and between the self-dose and the absorbed dose due to cross dose only ((B)). The self-dose for the right kidney (median (range)) was 4.3 Gy (1.6–9.7 ) and the left kidney 4.1 Gy (1.0–9.8). The cross-doses were 0.1 Gy (0.0–0.5) for both right and left kidney. The total absorbed doses were 4.4 (1.7–9.8 ) and 4.2 Gy (1.1–9.8) for the right and left kidney, respectively. Although the differences in absorbed dose between right and left kidney in the individual patients was sometimes large 0.2 Gy (−4.9–5.3 ), it was not statistically significant.
Figure 1. Absorbed doses to kidneys in 500 patients plotted as total dose versus self-dose (A), cross-dose versus self-dose (B).
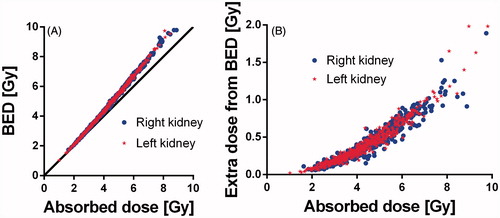
BED to the right kidney was 4.7 (1.7 – 11.6) and 4.4 Gy (1.0–11.8), respectively to the left kidney. In the BED is plotted versus the absorbed dose(A)) and also the extra contribution for the BED from the absorbed dose is plotted versus the absorbed dose ((B)). The extra contribution increases with increasing absorbed dose and was 0.4 (0.1–1.9) for the right kidney and 0.3 Gy, respectively (0.0–2.0) for the left kidney.
Figure 2. BED and absorbed doses to kidneys plotted as BED versus absorbed dose (A) and the contribution of BED that exceeds the absorbed dose versus the absorbed dose (B).
With a maximum tolerated absorbed dose of 23 Gy to the kidneys or 2 Gy to the bone marrow, over multiple treatment cycles assuming the same dose to both kidneys and bone marrow, the dose-limiting organ was the kidney in 97% of the patients. When a maximum tolerated BED of 38 Gy to the kidney or an absorbed dose of 2 Gy to the bone marrow was applied, the kidney was the dose-limiting organ in 87%. The maximum number of 7.4 GBq therapy cycles per patient was five (2–14) based on the absorbed dose limits 23 Gy to kidneys and 2 Gy to bone marrow and eight (3–20 ) cycles when based on the 38 Gy BED limit to the kidneys and 2 Gy absorbed dose to the bone marrow (). According to these results, by using these absorbed dose limits, about 2/3 of patients could receive more than the four cycles that has been considered a gold standard, while some patients (12%) would reach the dose limit after only 2–3 cycles. With the BED limit for kidneys combined with absorbed dose limit to bone marrow, more than 95% of the patients could receive more than four cycles, while a few patients (0.5%) would reach the dose limit after only three cycles.
Discussion
The clinical value of PRRT in patients with disseminated neuroendocrine tumors has been reported in several publications [Citation1,Citation2,Citation4,Citation9]. However, many issues remain to be solved in order to improve and personalize the therapy [Citation25]. There is a general agreement in the literature on the importance of individualized therapy using dosimetry as a tool to achieve an individual risk-assessment for sensitive organs [Citation26]. Kidney toxicity is a limiting factor for PRRT, especially when using 90Y [Citation1,Citation7,Citation15]. Renal toxicity with 177Lu-DOTATATE is low, although the absorbed dose threshold remains to be defined [Citation4,Citation26].
Dosimetry in 177Lu-DOTATATE PRRT is highly dependent on the correct input data. Important factors include the accuracy in the quantitative imaging, the timing of the measurements for a correct estimation of the time integrated activity concentration/activity and the accuracy of the absorbed dose calculations.
The main aim of the Uppsala group, working on 177Lu-DOTATATE PRRT dosimetry, has been dedicated to developing a clinically applicable and robust dosimetry protocol for solid organs based on 3-D imaging [Citation19–21]. 177Lu-DOTATATE PRRT dosimetry based on 2-D imaging suffers from problems when delineating healthy organ uptake due to overlaying uptake from tumors and physiological uptake in the bowel [Citation8,Citation27,Citation28] and is instead best used to provide input data for cross-dose calculations.
An earlier simulation study [Citation18], showed that the absorbed dose for the kidneys originate mainly from the organs themselves (self-dose) with only a few percent of the absorbed dose resulting from radiation from other organs (cross-dose) and therefore implying that the self-dose alone should represent a good estimate of the absorbed dose. This was verified in the vast majority of our patients, in whom the extra absorbed dose was less than 2%, the cross-dose was less than 10% in more than 97% of the patients and less than 20% in the whole group. For all the patients where more than 10% of the absorbed dose originated from cross-dose had an estimated absorbed dose of less than 3 Gy per cycle. In order to individualize 177Lu-DOTATATE PRRT it is important to keep in mind even for patients with low absorbed dose to the kidneys and high uptake in the surrounding organs that the self-dose still serves as a good estimate of the absorbed dose.
As shown in the BED was only slightly higher than the absorbed dose and the extra contribution due to the BED calculation increased with increasing absorbed dose. Since the α/β in this case was not determined for the specific BED calculation for kidneys during any PRRT, but rather for a more general radiation therapy administering multiple fractions per day, these results should be considered as approximations. Also the 38 Gy BED limit is not certain and really needs to be verified. Still, the calculations imply that for PRRT with 177Lu-DOTATATE the BED value is fairly similar to the absorbed dose value. In the present study, it was shown that from a dosimetry point of view, based on the absorbed dose and BED calculations, it should be safe for almost all patients to receive four treatment cycles of 7.4 GBq (). Furthermore our results imply that the majority of patients should be able to receive more than four treatments and this may improve their outcome, as indicated by a previous study in patients with pancreatic neuroendocrine tumors [Citation11] showing correlation between tumor shrinkage and absorbed dose in the tumors. Consequently, our results emphasize the need for a clinical study in which a higher dose limit to the kidneys is tested.
The absorbed dose from 177Lu-DOTATATE to solid organs due to cross-fire is generally low and can be neglected. To meet the recommendation of at most 20% extrapolated fractional contribution to the determination of kidney absorbed doses, the first imaging should preferably be performed no later than 8–12 h and the last measurement at least seven days after treatment start. According to our results, by using the 23 Gy absorbed dose to kidney and 2 Gy absorbed dose to bone marrow as limits, almost 2/3 of the patients could receive more than the standard therapy protocol of four cycles. By applying the 38 Gy BED limit for kidneys combined with 2 Gy absorbed dose limit to bone marrow, more than 95% of the patients could receive more than four cycles. Higher absorbed dose to tumor should improve therapy outcome, however such a regime would require absorbed dose calculations for the individual patient.
Acknowledgments
The authors want to thank the staff at the department of medical physics, endocrine oncology and nuclear medicine at Uppsala university hospital for their support. Erasmus Rotterdam is gratefully acknowledged for their supply of the peptide.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423.
- Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with (1)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–2135.
- Gabriel M, Andergassen U, Putzer D, et al. Individualized peptide-related-radionuclide-therapy concept using different radiolabelled somatostatin analogs in advanced cancer patients. Q J Nucl Med Mol Imaging. 2010;54:92–99.
- Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130.
- Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–2762.
- Williams LE, DeNardo GL, Meredith RF. Targeted radionuclide therapy. Med Phys. 2008;35:3062–3068.
- Bodei L, Cremonesi M, Ferrari M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–1856
- Valkema R, Pauwels SA, Kvols LK, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med. 2005;46:83S–91S.
- Bodei L, Cremonesi M, Grana C, et al. Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2004;31:1038–1046.
- Moll S, Nickeleit V, Mueller-Brand J, et al. A new cause of renal thrombotic microangiopathy: yttrium 90-DOTATOC internal radiotherapy. Am J Kidney Dis. 2001;37:847–851.
- Ilan E, Sandstrom M, Wassberg C, et al. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med. 2015;56:177–182.
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122.
- Konijnenberg MW. Is the renal dosimetry for [90Y-DOTA0,Tyr3]octreotide accurate enough to predict thresholds for individual patients? Cancer Biother Radiopharm. 2003;18:619–625.
- Konijnenberg M, Melis M, Valkema R, et al. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J Nucl Med. 2007;48:134–142.
- Barone R, Borson-Chazot F, Valkema R, et al. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med. 2005;46:99S–106S.
- Sabet A, Ezziddin K, Pape UF, et al. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur J Nucl Med Mol Imaging. 2014;41:505–510.
- Hindorf C, Glatting G, Chiesa C, et al. EANM dosimetry committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging. 2010;37:1238–1250.
- Ljungberg M, Sjogreen-Gleisner K. The accuracy of absorbed dose estimates in tumours determined by quantitative SPECT: a monte carlo study. Acta Oncol. 2011;50:981–989.
- Sandstrom M, Ilan E, Karlberg A, et al. Method dependence, observer variability and kidney volumes in radiation dosimetry of (177)Lu-DOTATATE therapy in patients with neuroendocrine tumours. EJNMMI Phys. 2015;2:24.
- Sandstrom M, Garske-Roman U, Granberg D, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33–41.
- Sandstrom M, Garske U, Granberg D, et al. Individualized dosimetry in patients undergoing therapy with (177)Lu-DOTA-D-Phe (1)-Tyr (3)-octreotate. Eur J Nucl Med Mol Imaging. 2010;37:212–225.
- Garske U, Sandstrom M, Johansson S, et al. Minor changes in effective half-life during fractionated 177Lu-octreotate therapy. Acta Oncol. 2012;51:86–96.
- Fleming JS. A technique for the absolute measurement of activity using a gamma camera and computer. Phys Med Biol. 1979;24:176–180.
- Thames HD, Ang KK, Stewart FA, et al. Does incomplete repair explain the apparent failure of the basic LQ model to predict spinal cord and kidney responses to low doses per fraction? Int J Radiat Biol. 1988;54:13–19.
- Prasad V, Bodei L, Kidd M, et al. Whither peptide receptor radionuclide therapy for neuroendocrine tumors: an einsteinian view of the facts and myths. Eur J Nucl Med Mol Imaging. 2014;41:1825–1830.
- Cremonesi M, Botta F, Di Dia A, et al. Dosimetry for treatment with radiolabelled somatostatin analogues. A review. Q J Nucl Med Mol Imaging. 2010;54:37–51.
- Garkavij M, Nickel M, Sjogreen-Gleisner K, et al. 177Lu-[DOTA0,Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer. 2010;116:1084–1092.
- Wehrmann C, Senftleben S, Zachert C, et al. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm. 2007;22:406–416.