Abstract
Aim: To correlate dose-volume histogram (DVH) parameters with appearance of grade ≥2 acute and late gastrointestinal toxicity of stereotactic body radiotherapy (SBRT) in patients with abdominopelvic solitary or oligometastatic disease outside the liver.
Material and methods: Acute and late bowel toxicity of 84 abdominopelvic oligometastatic patients was registered. A logistic regression was performed between different DVH parameters and presence of grade ≥2 acute and late toxicity. A Normal Tissue Complication Probability (NTCP) model was built with significant parameters to determine complication probabilities (CP).
Results: Thirteen (15%) of 84 patients experienced of grade ≥2 acute toxicity, while 8 (10%) reported late toxicity complications. A significant relationship was found for EQD2 (V30Gy, V40Gy, V50Gy and V65Gy) and grade ≥2 acute toxicity. Dmax and D2 were not significant. Late grade ≥2 toxicity was not significantly correlated with any DVH parameter. According to our NTCP model for V40Gy, an irradiated bowel volume of 10 cm3 of V40Gy resulted in CP of grade ≥2 acute toxicity of less than 10%. Local control was 87% at 2 years and 82% at 5 years. Overall survival was 61% at 2 years and 32% at 5 years.
Conclusions: After SBRT for abdominopelvic oligometastases, in general, the presence of acute and late toxicity was low. A significant relationship was found for V30Gy, V40Gy, V50Gy and V65Gy and grade ≥2 acute toxicity. We estimated acute complication probabilities per volume of irradiated bowel by V40Gy and V50Gy
Introduction
Stereotactic body radiotherapy (SBRT) is effective in several tumor sites such as early stage lung cancer, liver cancer and brain tumors [Citation1–3]. The main advantages of SBRT are the ability to deliver high doses to the tumors while sparing organs at risk (OAR) [Citation4]. However, the proximity of the radiosensitive bowel limits the use of stereotactic radiotherapy in the abdomen. With conventional radiotherapy, the current practice is to avoid irradiating the bowel with more than 50 Gy whenever possible. Baglan et al. published on rectal cancer patients, treated with concomitant radiation and 5-fluorouracil (5FU)-based chemotherapy, a model which predicted a low risk (around 10%) of Grade ≥3 acute small-bowel toxicity, for patients whose absolute volumes of small bowel receiving 5 to 40 Gy (V5–V40) were below 120–75 cm3 [Citation5,Citation6].
First reports of SBRT in pancreatic cancer reported high rates of late bowel toxicity using a fractionation scheme of 45 Gy in 3 fractions [Citation7]. The same group later reported 4% of grade 3 toxicity in 64 patients treated for abdominal lymph node oligometastases with similar fractionation [Citation8]. Later studies [Citation9–12] have used milder fractionations and have reported very low grade ≥3 late toxicity rates. Researchers from Stanford University [Citation13,Citation14] employed 25 Gy in one fraction on the treatment of pancreatic cancer, and demonstrated a correlation between DVH parameters Dmax, V15Gy, V20Gy and the onset of grade ≥2 late duodenal toxicity. Bae et al. [Citation15], using a median dose of 45 Gy in 3 fractions, reported that dose-volume parameters such as V25Gy, V30Gy, V35Gy and Dmax were found to be good predictors of grade ≥3 late gastroduodenal toxicity. In studies of non-stereotactic radiotherapy for rectal cancer patients [Citation5,Citation16–18], a correlation between V15Gy and V25Gy with presence of acute gastrointestinal toxicity grade ≥3 was reported.
There is still a lack of clear consensus concerning the relation of DVH parameters to toxicity for SBRT. None of the studies cited above have converted their reported doses to biologically equivalent doses, neither have they reported the odds of toxicity or complication probabilities per volume irradiated. The goal of the current study is to report the correlation between acute and late grade ≥2 bowel toxicity and various DVH parameters after stereotactic radiotherapy for patients with solitary or oligometastatic tumors in the abdomen or pelvis, and calculate the odds of toxicity per cm3 and the subsequent complication probabilities. Secondary endpoints were local control, survival and late toxicity.
Material and methods
Eighty-four patients with solitary or oligometastatic abdominopelvic tumors were treated with SBRT between 2007 and 2014. Patients with metastases in the liver were excluded. Depending on tumor size and location, different hypofractionated treatment schedules were used. Fifty-three patients were treated with 48 Gy in 6 fractions, while 12 received 45 Gy in 5 fractions. The remaining 17 patients were treated with different schedules (). The study was approved by the institutional review board (IRB).
Table 1. Treatment schedules.
Continuous tracking of tumor motion and automatic adjustment of beam aim were used for each treatment. Thus, a narrow uncertainty margin from GTV to PTV, 2–3 mm, was used. For tumors that moved with respiration, clips were placed in the tumor and tracked over treatment. The stomach, duodenum and bowel were contoured as separate structures, but for analysis were summed together. The total dose was prescribed to cover, at least, 95% of the volume of the PTV, equaled to the 75–85% (median: 80%) of the maximum dose, delivered to the PTV and was prescribed to the outer line of the PTV. However, to spare organs at risk (such as bowel or spinal cord), a lower coverage was accepted. To analyze the data, all doses were converted into an equivalent dose of 2 Gy (EQD210 and EQD23). An α/β of 10 Gy was assumed for acute toxicity and 3 Gy for late toxicity. Acute and late bowel toxicity was scored retrospectively using the Common Terminology Criteria for Adverse Events v3.0 (CTCAEv3). Acute bowel symptoms were nausea and/or diarrhea and pain. Late complications were chronic abdominal pain, diarrhea, dysuria and urinary incontinence. Toxicity within 6 months of treatment was scored as acute bowel toxicity, and after 6 months it was scored as late bowel toxicity. A logistic regression was performed to correlate the presence or not of acute and late grade ≥2 bowel toxicity with different DVH parameters. Dose volume parameters investigated were as follows: D2, Dmax, V30Gy, V40Gy, V50Gy, V65Gy, V80Gy, V90Gy and V100Gy. Normal tissue control probability (NTCP) was calculated based on logistic regression model [Citation19,Citation20]. The most significant parameter of regression was fitted into an NTCP model curve. The odds were obtained and introduced into the equation ln(odds) for calculation of log odds:
(1)
We built our NTCP with the following equation:
(2)
A Logistic model was applied to obtain complication probabilities by the amount of volume encompassed by a certain dose. Statistical analysis was done using SPSS (IBM Corp. 2012, Version 21.0. Armonk, NY, USA) and Microsoft Excel 2010.
Local control, overall and disease free survival were calculated by Kaplan–Meier method. Local control was calculated from the start of radiotherapy until the diagnosis of a local recurrence, based on the modified RECIST criteria [Citation21]. Overall survival was measured from the start of radiotherapy until death from any cause.
None of the patients received prophylactic corticosteroids or antiemetics. The interval between the last chemotherapy and SBRT was at least 3 weeks. As a staging CT after the last chemotherapy is usually done after 2 weeks. Besides that, the patients were all discussed in a tumor board, usually 1 week after the CT scan and then referred to the radiation department. So the time between the last chemotherapy and the radiation was at least 4 weeks.
Results
Of 84 patients treated, 65 had a tumor in the para-aortic or iliac lymph nodes, 19 had metastases in the abdomen or pelvis. Median age was 67 years (range 27–87). Median follow-up was 19 months (range 4–22). Fifty patients (59%) were female, 34 (41%) male. Metastases were from primary colorectal cancer in 24 patients, gynecologic cancer for 23, 7 patients had bladder cancer, 6 sarcoma, 5 lung cancer, 3 renal cell cancer, and 3 cancer of unknown primary (CUP), as well as 13 other tumors.
No grade 4 or 5 toxicity was reported. In total, 13 patients (15%) complained of grade ≥2 acute gastro-intestinal (GI) toxicity, 10 patients complained of grade ≥2 nausea, 3 patients presented with grade 3 nausea and 7 patients presented with grade 2 toxicity. Four patients complained of grade ≥2 acute diarrhea. The median V40Gy was 13 cm3 in 77 patients. The median volumes (cm3) and doses are shown in and . Significant correlations were found between the presence of grade ≥2 acute toxicity and DVH parameters (). The odds of toxicity per cm3 for the other significant parameters were 1.3% for V30Gy, a 6.6% for V50Gy and 23.4% for V65Gy. We found no significant correlations between D2 (p = .197) and Dmax (p = .638), or for high-dose volumes such as V80Gy, V90Gy and V100Gy.
Table 2. DVH parameters of the bowel.
Table 3. D2 and Dmax of the bowel.
Table 4. Results of univariate analysis by logistic regression. Odds of acute and late toxicity for each DVH parameter.
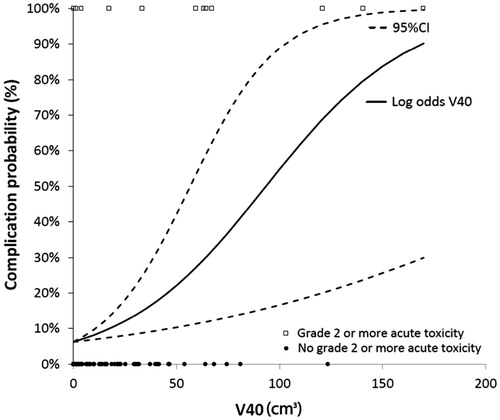
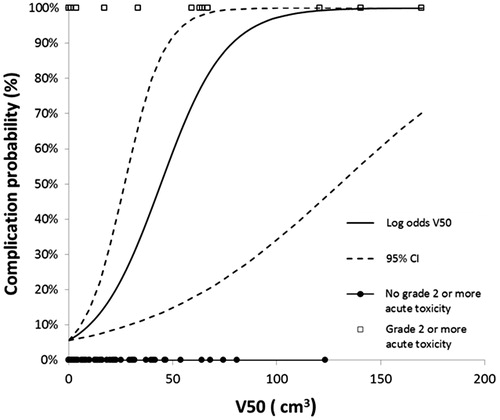
We built an NTCP model based on V40Gy and V50Gy. According to our NTCP model for V40Gy, an irradiated bowel volume of 10 cm3 resulted in CP of grade ≥2 acute toxicity of less than 10% (). An acute bowel complication probability of 51%, corresponded to a V50Gy of 45 cm3 ().
Late toxicity grade ≥2 was reported in 8 (10%) patients. Three patients reported grade 3 chronic pain, five complained of grade 2 chronic pain and six patients presented grade 1 chronic pain. There were 4 patients with grade 1 chronic diarrhea, and one patient with grade 1 fecal incontinence. No patient presented urinary incontinence. No significant correlation between volumetric parameters and the presence of chronic toxicity grade ≥2 was found in the regression analysis ().
The estimated overall survival rate was 61% (SE 0.05) at 2 years and 32% (SE 0.06) at 5 years. The median survival was 37 months (95% CI, 26.5–47.6 months). No significant difference in survival was found between patients with or without grade ≥2 toxicity (log rank test, p = .603). Local control was found in 87% of patients at 2 years (SE 0.046) and in 82% at 5 years (SE 0.051). Disease-free survival (DFS) was 44% (SE 0.058) at 2 years and 28% at 5 years. The median DFS was 19 months (95% CI, 10.9–27.6).
Discussion
The present series reports 15% of acute bowel toxicity grade ≥2, which is higher compared to other studies, although grade 3 toxicity was only seen in 4% of the patients. Local control was comparable to other series with equivalent fractionation [Citation9–12,Citation22]. The parameters V30Gy, V40Gy, V50Gy and V65Gy were significantly correlated with grade ≥2 toxicity. An increase of 3% in the odds of toxicity was found for per cm3 encompassed by V40Gy. The increased odds of toxicity per cm3 for the other significant parameters were 1.3% for V30Gy, 6.6% for V50Gy and 23.4% for V65Gy.
The values of V40Gy were introduced into a logistic NTCP model to estimate complication probabilities (CP) per volume irradiated. We found a less than 10% rate of CP when V40Gy were less than 10 cm3. A recent publication from Cooper hospital [Citation23] demonstrated that 21 Gy in 3 fractions to 5 cm3 of small bowel was associated with a low rate of toxicity. The group estimated a risk of 6.5% for grade 3 or higher complications at 5 years. Applying it to the present model, 21 Gy in 3 fractions corresponds to an EQD210 of 30 Gy; so considering an increase of 1.3% in the odds of toxicity per cm3, and assuming a volume of 5 cm3 (1.3% × 5 cm3), a similar estimation of 6.5% results. The differences are, that the present study reports grade ≥2 acute and late toxicity (not just grade 3 toxicity) and we estimated the odds of toxicity.
Based on the regression results, the parameters Dmax and D2 did not show a significant correlation. In fact, the median Dmax for patients with complications was not different compared to the patients with no complications (82 Gy vs 81 Gy). Indeed, the Dmax in dose planning is simply the highest dose found in any voxel of the planning grid that is inside the bowel; this Dmax point could also be located in the stools and therefore not predict toxicity. The median D2 of patients with grade ≥2 acute toxicity was 45 Gy, while for patients without grade ≥2 acute toxicity was 35 Gy.
A study from Stanford University [Citation13] reported on 73 patients with locally advanced pancreatic cancer (LAPC) treated by concomitant chemoradiation followed by a boost of 25 Gy in one fraction of SBRT. They obtained six- and 12-month actuarial toxicity rates of 11% and 29%, respectively. The parameters Dmax, V15Gy and V20Gy were all significantly correlated with duodenal toxicity (p < .05). A V15Gy ≥9.1 cm3 and V20Gy >3.3 cm3 yielded duodenal toxicity rates of 52%, compared to 11% (p = .002) for patients with V15Gy ≤9.1 cm3 and V20Gy <3.3 cm3, respectively. Moreover, Dmax ≥23 Gy and Dmax <23 Gy showed toxicity rates of 49% and 12%, respectively (p = .004). The same group [Citation14] described a trend towards the appearance of severe toxicity when the duodenal volume was encompassed by the V12.5Gy (≥50% isodose line) (p = .13). Bae et al. [Citation15] using a median dose of 45 Gy in 3 fractions, for the treatment of abdominal oligometastases, found that V25Gy >20 cm3, V30Gy >5 cm3 and V35Gy>1 cm3 were predictors of severe gastroduodenal toxicity. They reported that gastroduodenal Dmax values of 35 Gy and 38 Gy were associated with a probability of 5% and 10%, respectively, of grade ≥3 gastroduodenal toxicity.
In non-stereotactic treatments, certain dose-volume parameters have been found to be predictive of toxicity. Baglan et al. [Citation5] reported a threshold model to predict acute bowel toxicity in rectal cancer patients, treated with concomitant chemoradiation. They identified V15Gy as an important predictor of toxicity with a cutoff of 120 cm3. These findings were confirmed by Gunlaugsson et al. who observed a point threshold effect in patients treated with concomitant chemoradiation, whereby patients with an absolute V15Gy <150 cm3 experienced lower risk of grade ≥2 acute toxicity [Citation17]. Banerjee et al. [Citation16] concluded that the greatest sensitivity for predicting toxicity was associated with V15Gy and V25Gy. In their protocol, the peritoneal space was delineated. A threshold of V15Gy <275 cm3 and a peritoneal space V15Gy <830 cm3 were associated with <10% risk of grade ≥3 acute toxicity. Robertson et al. [Citation20] showed significant correlations between grade 3 bowel toxicity and V15Gy. Tho et al. [Citation24] reported in patients treated for rectal cancer that median small bowel volume differed significantly between patients experiencing Grade 0–1 and Grade 2–4 diarrhea, respectively (p ≤ .05).
Grade ≥2 late toxicity in the current study was higher (10%) compared to other oligometastases series, where the rate of reported late toxicity is very low. In the present study, the constraints used for bowel were Dmax = 35 Gy in 5 fraction treatment, and 0.5 cc were allowed to have more dose in any of the examined organs.
Milano et al. [Citation9,Citation25] and other series [Citation10–12] showed no grade >3 toxicity using fractionations equivalent to BED3 ≤180. Other studies that have used fractionations equivalent to BED3 >180, have reported cases of non-fatal grade 4 toxicity [Citation7,Citation8,Citation26]. We described only 3 patients with grade 3 toxicity and no patients with grade 4 or 5 toxicity.
In the present series, most patients were treated with doses per fraction <10 Gy. It is therefore assumed that the Linear quadratic (LQ) and EQD2 model can be used to compare doses across these treatment plans. With the recalculation to an EQD2, we were able to recalculate the different fractionation doses to an equivalent dose (e.g. V40, V50, …) and therefore, we were not only able to compare the different fractionation schedules of SBRT but also with conventional RT, however, the proper method is not yet fully elucidated. Future studies will answer the question if the LQ model and EQD2 hold up properly for high hypofractionation [Citation20,Citation27].
Conclusions
In conclusion, after stereotactic radiotherapy for abdominopelvic oligometastases, in general, the presence of acute and late toxicity was low. Only thirteen patients (15%) presented grade ≥2 acute toxicity. Late toxicity grade ≥2 was present in only 8 patients. The volume irradiated by V30Gy to V65Gy was correlated with acute grade ≥2 bowel toxicity. The parameters D2 and Dmax were not significant. We estimated acute complication probabilities per volume irradiated by different DVH parameters. Further studies are necessary to validate these parameters as surrogate for acute and late bowel toxicity.
Disclosure statement
We declare no conflict of interest.
References
- Mendez Romero A, Wunderink W, van Os RM, et al. Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int J Radiat Oncol Biol Phys. 2008;70:1447–1452.
- Mokhles S, Nuyttens JJ, Maat AP, et al. Survival and treatment of non-small cell lung cancer stage I-II treated surgically or with stereotactic body radiotherapy: patient and tumor-specific factors affect the prognosis. Ann Surg Oncol. 2015;22:316–323.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076.
- Prevost JB, Voet P, Hoogeman M, et al. Four-dimensional stereotactic radiotherapy for early stage non-small cell lung cancer: a comparative planning study. Technol Cancer Res Treat. 2008;7:27–34.
- Baglan KL, Frazier RC, Yan D, et al. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176–183.
- Robertson JM, Lockman D, Yan D, et al. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:413–418.
- Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53.
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830.
- Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–658.
- Ponti E, Ingrosso G, Carosi A, et al. Salvage stereotactic body radiotherapy for patients with prostate cancer with isolated lymph node metastasis: a single-center experience. Clin Genitourin Cancer. 2015;13:e279–e284.
- Rwigema JC, King C, Wang PC, et al. Stereotactic body radiation therapy for abdominal and pelvic oligometastases: dosimetric targets for safe and effective local control. Pract Radiat Oncol. 2015;5:e183–e191.
- Scorsetti M, Bignardi M, Alongi F, et al. Stereotactic body radiation therapy for abdominal targets using volumetric intensity modulated arc therapy with RapidArc: feasibility and clinical preliminary results. Acta Oncol (Stockholm, Sweden). 2011;50:528–538.
- Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426.
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686.
- Bae SH, Kim MS, Cho CK, et al. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys. 2012;84:e469–e474.
- Banerjee R, Chakraborty S, Nygren I, et al. Small bowel dose parameters predicting grade >/= 3 acute toxicity in rectal cancer patients treated with neoadjuvant chemoradiation: an independent validation study comparing peritoneal space versus small bowel loop contouring techniques. Int J Radiat Oncol Biol Phys. 2013;85:1225–1231.
- Gunnlaugsson A, Kjellen E, Nilsson P, et al. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol (Stockholm, Sweden). 2007;46:937–944.
- Roeske JC, Bonta D, Mell LK, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69:201–207.
- Agresti A, Finlay B. Statistical methods for the social sciences. 4th ed. Upper Saddle River (NJ): Pearson Prentice Hall; 2009.
- Robertson JM, Sohn M, Yan D. Predicting grade 3 acute diarrhea during radiation therapy for rectal cancer using a cutoff-dose logistic regression normal tissue complication probability model. Int J Radiat Oncol Biol Phys. 2010;77:66–72.
- Nishino M, Jagannathan JP, Ramaiya NH, et al. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–289.
- Jereczek-Fossa BA, Ronchi S, Orecchia R. Is Stereotactic Body Radiotherapy (SBRT) in lymph node oligometastatic patients feasible and effective?. Rep Pract Oncol Radiother. 2015;20:472–483.
- LaCouture TA, Xue J, Subedi G, et al. Small bowel dose tolerance for stereotactic body radiation therapy. Semin Radiat Oncol. 2016;26:157–164.
- Tho LM, Glegg M, Paterson J, et al. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys. 2006;66:505–513.
- Milano MT, Katz AW, Okunieff P. Patterns of recurrence after curative-intent radiation for oligometastases confined to one organ. Am J Clin Oncol. 2010;33:157–163.
- Kang JK, Kim MS, Kim JH, et al. Oligometastases confined one organ from colorectal cancer treated by SBRT. Clin Exp Metastasis. 2010;27:273–278.
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19.