Abstract
Background: Neoadjuvant chemotherapy represents a new treatment approach to locally advanced colon cancer. The aim of this study was to analyze the ability of tumor–stroma ratio (TSR) to predict disease recurrence in patients with locally advanced colon cancer treated with neoadjuvant chemotherapy.
Material and methods: This study included 65 patients with colon cancer treated with neoadjuvant chemotherapy in a phase II trial. All patients were planned for three cycles of capecitabine and oxaliplatin before surgery. Hematoxylin and eosin stained tissue sections from surgically resected primary tumors were sampled and analyzed by conventional microscopy. Patients were divided into stroma-high (>50%, i.e. TSR low) and stroma-low (≤50%, i.e. TSR high) for the comparison with clinical data.
Results: A low TSR was found in 47% of the surgically resected primary tumors and correlated to a significantly higher T- and N-category compared, to tumors with a high TSR (p < .01). A low TSR was also significantly associated with disease recurrence (p = .008), translating into significant differences in disease free survival (DFS) and overall survival, p < .002. The 5-year DFS rate for patients with a low TSR was 55%, compared to 94% in the group of patients with a high TSR.
Conclusions: TSR assessed in the surgically resected primary tumor from patients with locally advanced colon cancer treated with neoadjuvant chemotherapy provides prognostic value and may serve as a relevant parameter in selecting patients for post-operative treatment.
Introduction
A considerable fraction (20–50%) of patients operated for stage II and III colon cancer experience disease recurrence, typically within two or three years after surgery [Citation1]. Early identification of the patients at risk is desirable and could spare many from ineffective adjuvant chemotherapy [Citation2].
Multiple putative prognostic markers have been assessed in this context over the last decades. Only a few, however, are considered suitable for guiding the clinicians and the patients in the decision on adjuvant chemotherapy [Citation3]. Assessing the ratio between adenocarcinoma cells and stromal cells in the primary tumor, i.e. tumor–stroma ratio (TSR), has gained increasing interest over the last 10 years due to the simplicity and the potential clinical value of this marker. It is a fast and cost efficient estimate based on routine diagnostic hematoxylin and eosin (H&E) stained tissue sections. Most studies regarding the technical aspects of TSR scoring provide kappa-values pointing to at least moderate, but mostly substantial and even optimal reproducibility. The current literature unanimously points toward TSR as a prognostic marker in patients operated for colon cancer [Citation4–10], although results from larger population based cohorts are still awaited. A high amount of stroma (low TSR) is related to a poor prognosis and may also influence the delivery of chemotherapeutics to the cancer cells.
Neoadjuvant chemotherapy is currently being investigated in patients with locally advanced colon cancer and may represent a new treatment approach ensuring early systemic treatment of minimal metastatic disease [Citation11]. The initial phase II experience indicates that a substantial proportion of these patients may convert to a low risk status at the time of operation, eliminating the need for adjuvant chemotherapy [Citation12]. Whether TSR after exposure to neoadjuvant chemotherapy still holds prognostic value after the operation has never been investigated before.
The aim of this study was to analyze the ability of TSR to predict disease recurrence in patients with locally advanced colon cancer treated with neoadjuvant chemotherapy.
Material and methods
Reporting in this study is in accordance with the REMARK criteria [Citation13].
Study population
This translational study refers to a previous phase II trial of neoadjuvant chemotherapy in patients with locally advanced colon cancer [Citation12]. The present study population represents 65 of the 71 patients who completed neoadjuvant chemotherapy followed by operation between August 2010 and September 2013. The reasons for excluding six patients were lack of tissue specimens in five cases and death a few days after surgery in one case. All patients were diagnosed with locally advanced, resectable colon cancer as detected by computed tomography (CT) scans. Patients with documented wild-type KRAS, BRAF and PIK3CA status received supplemental panitumumab, while patients with any mutation in the above genes, or unknown mutational status, received neoadjuvant chemotherapy only. All patients were ≥18 years, of performance status (PS) ≤ 2 and had a T3 tumor with extramural tumor invasion (ETI) > 5 mm or a T4 tumor based on CT scans. Selection criteria and follow-up have previously been specified [Citation12]. Patients were followed according to the protocol for up to three years post-operatively. This period was extended to five years for the present study to include survival data (dead/alive) and histopathologically verified recurrence (yes/no) of all included patients. Data were recorded according to good clinical practice. The clinical study, as well as the present translational research was approved by the Regional Scientific Ethical Committee and the Danish Data Protection Agency (S-20100014 and S-20160031) and written informed consent was obtained from all patients enrolled in the clinical study (ClinicalTrials.gov NCT01108107). The tissue used for research was confirmed not to be refused in the Danish Registry of Human Tissue Utilisation.
CT scanning
CT scanning was performed using a 64-channel multidetector. Effective dose: approximately 8–9 mSv. Images were obtained with breath-hold technique after intravenous injection of iomeprol (Iomeron, Bracco, Milan, Italy). Three mm CT images were transferred to and stored within the Picture and Archive Communication System (PACS). The CT images were evaluated by a certified subspecialist in abdominal radiology. Multiplanar reconstruction was performed on the PACS.
Treatment
Treatment consisted of neoadjuvant capecitabine 1000 mg/m2 orally twice daily on days 1 through 14 (28 doses) in a 21-day cycle, and oxaliplatin 130 mg/m2 as a two-hour intravenous infusion on day 1. Panitumumab 9 mg/kg on day 1 of the 21-day cycle was added to the neoadjuvant treatment for patients with wild-type mutational status. Tumor resection was performed three weeks after the last cycle of neoadjuvant chemotherapy. Patients fulfilling the criteria for adjuvant chemotherapy according to the Danish Colorectal Cancer Group (DCCG) criteria (http://dccg.dk/retningslinjer/20150806/2015_AdjKemoColon.pdf) (lymph node metastases, pT4 tumor, acute surgery due to bowel obstruction, neuronal or vascular invasion, high malignancy grade, less than 12 lymph nodes removed) received an additional five cycles of the same chemotherapy regimen. Patients not fulfilling the criteria (converted from a high risk group requiring adjuvant chemotherapy to low risk not requiring additional treatment) received no further treatment. This conversion rate and tumor regression grade (TRG) as evaluated on the operative specimens according to Mandard et al.’s [Citation14] were used to estimate the effect of neoadjuvant chemotherapy. This routine estimation was performed by one observer. The TRG scores spanned from 1, i.e. pathological complete response with no residual tumor, to 5, referring to no response. All patients were followed as previously specified [Citation12].
Tumor–stroma ratio
Tissue samples consisting of 5 µm thick H&E stained histologic sections from the diagnostic biopsy and from the deepest invasive part in the bowel wall of the primary tumor surgical specimen (slides used in routine pathology to determine T-category) were applied for TSR assessment. Areas with the seemingly largest amount of stroma were selected by conventional microscopy using a 2.5× or 5× objective. Subsequently, an area containing both tumor and stromal tissue in the vision-site was selected using a 10× objective. Tumor cells were present at all borders of the image field selected (north-east-south-west) for TSR semi-quantification.
TSR was estimated by one observer blinded to the clinical data. Scoring percentages were semi-quantitatively reported per tenfold (10%, 20%, 30%, etc.). For statistical analyses, stromal ratio groups were divided into stroma-high (>50%, i.e. TSR low) and stroma-low (≤50%, i.e. TSR high) as previously determined to have maximum discriminative power in the standard setting of upfront surgery [Citation10].
Statistics
Differences between proportions were evaluated using Fisher’s exact test. Disease free survival (DFS) was defined as the time from operation to the first documented tumor recurrence or death of any cause. Overall survival (OS) was calculated as the time from operation until death of any cause. All statistical calculations were performed using the NCSS statistical software (NCSS Statistical Software, version 2007, Kaysville, UT, USA). p Values < .05 were considered significant, and all tests were two-sided.
Results
Patient characteristics
Patient characteristics are summarized in . At the time of data analysis, i.e. at a median follow-up of 5.3 years, disease recurrence had occurred in 15 patients.
Table 1. Clinical and pathoanatomic characteristics, N = 65.
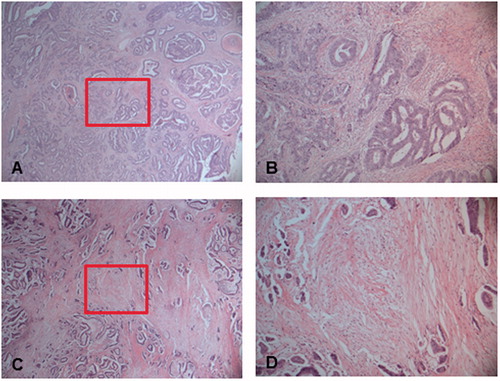
Representative images of tumors with high stroma content (low TSR) and low stroma content (high TSR) are shown in .
Figure 1. Representative images of a tumor with a low stroma content (high TSR) at (A) 25× and (B) at 100×, and a tumor with a high stroma content (low TSR) at (C) 25× and (D) at 100×. The boxes outline the sections illustrated in (B) and (D), respectively. Tumor cells were to be present at all borders of the selected image field (north-east-south-west).
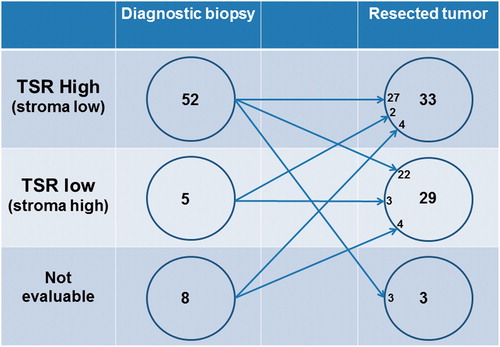
Paired diagnostic biopsies and resected primary tumors were not available for analysis in all patients. Assessment of TSR was possible in 57 (88%) of the diagnostic biopsies and 62 (95%) of the surgically resected primary tumors. The distribution is illustrated in .
Figure 2. Illustration of the sample distribution between biopsies and resected tumors and the scoring results from the tumor–stroma ratio (TSR) assessment.
The relationship between TSR in the surgically resected tumors and pathoanatomical characteristics is demonstrated in . A low TSR was significantly (p < .03) associated with perineural and vascular invasion, pT4 tumors, and lymph node metastases.
Table 2. Relationship between tumor–stroma ratio and pathoanatomic characteristics, N = 62.
Tumor–stroma ratio and effect parameters
A significant relationship was found between a low TSR in the surgically resected primary tumor and lack of treatment response assessed by TRG score (p = .0118) and risk group conversion rate (p = .0009) ().
Table 3. Relationship between tumor–stroma ratio and effect of neoadjuvant chemotherapy.
The potential challenge of comparing the two stromal-based methodologies (i.e. TSR and TRG) is illustrated in .
Recurrence and prognosis
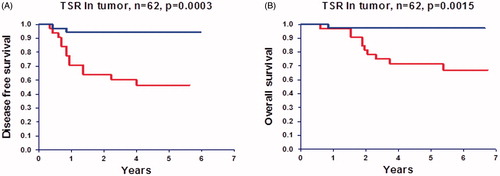
A significantly higher proportion of patients with low compared to high TSR experienced disease recurrence; 13/29 and 2/33, respectively, p = .008. The positive and negative predictive values of a low TSR were 45% (13/29) and 94% (31/33), respectively. This relationship with recurrence is translated into a significant difference in DFS and OS as illustrated in . The 5-year DFS rate in patients with a low TSR was 55% compared to 94% in the group with a high TSR.
Discussion
Neoadjuvant chemotherapy represents a new treatment approach in patients with locally advanced colon cancer. In the present study, TSR was assessed the first time in the neoadjuvant setting demonstrating an association with clinically relevant end points, and thus arguing for a role as a future biomarker in this setting.
We analyzed TSR in the diagnostic biopsies and the surgically resected primary tumor. The diversity of the TSR score in the biopsies was limited, in that 52 out of 57 biopsies (91%) were scored as TSR high, preventing meaningful comparison with the clinical data. To the best of our knowledge, this is the first report on TSR assessed in diagnostic biopsies and it is likely that the limited tissue area available in the biopsies is not adequate for the evaluation.
A low TSR in the surgically resected primary tumors was detected in 47% of the patients, a ratio comparable to the latest studies in the literature [Citation4,Citation5] although the initial studies generally indicated a lower frequency (24–29%) of ‘low TSR’ [Citation6–10]. A possible influence of neoadjuvant chemotherapy on tumor tissue composition may explain, at least partly, this difference.
Our results document a low TSR to be associated with a significantly higher risk of vascular and perineural invasion together with a higher T- and N-category compared to tumors with a high TSR. These observations corroborate previous findings [Citation4,Citation6–8,Citation10], and thus persist after neoadjuvant chemotherapy.
We also investigated whether, in the surgically resected primary tumor TSR was related to treatment effect. A significant relationship was found between TSR and TRG and the clinical down staging (from locally advanced tumor before treatment to not fulfilling the requirements for adjuvant treatment after the operation) indicating in both cases poor response to neoadjuvant chemotherapy in patients with a low TSR.
TRG and TSR do not reflect the same situation. TRG is based on the whole tumor and TSR on a selected hotspot as illustrated in . The existence of only one carcinoma focus in an otherwise fibrotic tumor, i.e. TRG 3, is not necessarily reflected by a low TSR. Even though the rest of the tumor is fibrotic, TSR may still be high. However, certain TRG 3 (and TRG 2) tumors may contain only very few scattered carcinoma cells rendering TSR assessment inadequate. Currently, there are no other studies addressing the possible predictive value of the TSR in relation to chemotherapy in colorectal cancer (CRC), but the present findings are plausible given the association with T- and N-category at the time of surgery.
Figure 4. Disease free survival (A) and overall survival (B) curves according to tumor–stroma ratio (TSR) in the surgically resected tumor. Upper curves represent patients with a high TSR (stroma low, N = 33) and lower curves patients with a low TSR (stroma high, N = 29).
We also found a convincing relationship between TSR and disease recurrence, and consequently prognosis. Patients with a high TSR had a very low risk of disease recurrence, which was only seen in two of these patients. The result was a negative predictive value of 94% indicating that in this setting TSR assessment in the surgically resected primary tumor may serve as a biomarker of clinical relevance. With only 6% of the patients in this group experiencing disease recurrence, one could argue that this group of patients should not be treated with adjuvant chemotherapy at all. In comparison, a recurrence rate of 6% is less than what is expected in stage I colon cancer where adjuvant chemotherapy is not recommended. Of the patients with a high TSR, 36% were not risk group converted by neoadjuvant treatment and consequently treated post-operatively with adjuvant chemotherapy. The reason for high stroma content being prognostically unfavorable is probably multifaceted, but increasing evidence suggests cellular components of the stroma to be triggering the epithelial–mesenchymal transition in the invasive zone of the tumor and hence increasing the risk of metastatic spread [Citation15].
The current literature on the clinical potential of TSR assessment in CRC is rather convincing and offers several studies indicating a prognostic impact in stages I–III colon cancer comparable to the present study in the neoadjuvant setting. Additionally, the simplicity of semi-quantifying TSR, freely available through routine diagnostics, strengthens its potential as a biomarker. Results from large population based cohorts, however, are still awaited. The same applies to studies on the possible predictive value and especially, on the value of the TSR technique in rectal cancer. Validation of the current results is ongoing in a randomized phase III trial, NeoCol (https://clinicaltrials.gov/ct2/show/NCT01918527?term=NeoCol&cond=Colon+Cancer&rank =1), offering neoadjuvant chemotherapy to patients with locally advanced colon cancer.
In conclusion, this first study in the neoadjuvant setting demonstrated that TSR assessed in the surgically resected primary tumor from patients with locally advanced colon cancer treated with neoadjuvant chemotherapy provides prognostic value and may serve as a relevant parameter in selecting patients for post-operative treatment.
Acknowledgments
We are very thankful for the technical assistance provided by Birgit Roed Sørensen and Margit Søgaard Jakobsen, and for the linguistic editing provided by Karin Larsen.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Bockelman C, Engelmann BE, Kaprio T, et al. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16.
- O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–3388.
- Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:64–72.
- Pelt GW, Hansen TF, Bastiaannet E, et al. Stroma-high lymph node involvement predicts poor survival more accurately for patients with stage III colon cancer. J Med Surg Pathol. 2016;1:116. doi:10.4172/jmsp.1000116.
- Vogelaar FJ, Pelt GW, van Leeuwen AM, et al. Are disseminated tumor cells in bone marrow and tumor–stroma ratio clinically applicable for patients undergoing surgical resection of primary colorectal cancer? The Leiden MRD study. Cell Oncol. 2016;39:537–544.
- Park JH, Richards CH, McMillan DC, et al. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol. 2014;25:644–651.
- Huijbers A, Tollenaar RA, Pelt GW, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol. 2013;24:179–185.
- West NP, Dattani M, McShane P, et al. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer. 2010;102:1519–1523.
- Mesker WE, Liefers GJ, Junggeburt JM, et al. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I–II colon cancer patients. Cell Oncol. 2009;31:169–178.
- Mesker WE, Junggeburt JM, Szuhai K, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387–398.
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152–1160.
- Jakobsen A, Andersen F, Fischer A, et al. Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol. 2015;54:1747–1753.
- McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387–391.
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686.
- Lambert A, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691.