Abstract
Background: Whole-brain radiotherapy (WBRT) has been the standard of care for multiple NSCLC brain metastases but due to its toxicity and lack of survival benefit, its use in the palliative setting is being questioned.
Patient and methods: This was a single institution cohort study including brain metastasized lung cancer patients who received WBRT at Karolinska University Hospital. Information about Recursive Partitioning Analysis (RPA) and Graded Prognostic Assessment (GPA) scores, demographics, histopathological results and received oncological therapy were collected. Predictors of overall survival (OS) from the time of received WBRT were identified by Cox regression analyses. OS between GPA and RPA classes were compared by pairwise log rank test. A subgroup OS analysis was performed stratified by RPA class.
Results: The cohort consisted of 280 patients. RPA 1 and 2 classes had better OS compared to class 3, patients with GPA <1.5 points had better OS compared to GPA≥ 1.5 points and age >70 years was associated with worse OS (p< .0001 for all comparisons). In RPA class 2 subgroup analysis GPA ≥1.5 points, age ≤70 years and CNS surgery before salvage WBRT were independent positive prognostic factors.
Conclusions: RPA class 3 patients should not receive WBRT, whereas RPA class 1 patients should receive WBRT if clinically indicated. RPA class 2 patients with age ≤70 years and GPA ≥1.5 points should be treated as RPA 1. WBRT should be omitted in RPA 2 patients with age >70. In RPA 2 patients with age ≤70 years and GPA <1.5 points WBRT could be a reasonable option.
Introduction
Dissemination in the CNS occurs in up to 44% of patients with advanced nonsmall cell lung cancer (NSCLC), more frequently in lung adenocarcinoma [Citation1]. Whole-brain radiotherapy (WBRT) has been the standard of care for multiple NSCLC brain metastases (BM) for many years [Citation2]. Nonetheless, a substantial number of patients may experience considerable neurocognitive sequelae and declines in quality of life caused by WBRT, such as moderate-to-severe dementia and verbal memory decline [Citation3–5].
The recursive partitioning analysis (RPA) classification system published in 1997, which divided patients with brain metastases in three prognostic classes according to age, control of primary tumor, performance status measured according to Karnofsky Performance Scale (KPS) and the presence of extracranial disease [Citation6], was the first clinically important scoring system for BM patients receiving WBRT. Thereafter several prognostic scoring systems have been proposed for patients receiving WBRT or Stereotactic Radiosurgery (SRS) (or both), adding parameters such as extracranial tumor control, interval between tumor diagnosis and WBRT start, number and volume of brain metastases, in relation to neurological outcomes and survival [Citation7–12]. Sperduto et al introduced the new graded prognostic assessment (GPA) in 2008 comparing RPA, Basic Score for Brain Metastases (BSBM) and Score Index for Radiosurgery (SIR) in 1960 patients from the RTOG database. GPA was as accurate as the RPA as prognostic tool and performed better than the other indices [Citation13]. GPA has thereafter been validated in other cohorts [Citation14,Citation15].
Despite the availability of diverse scoring systems, there still is a lack of consensus regarding which clinical factors have the major impact in treatment decision-making concerning the use of WBRT in brain metastatic lung cancer, especially in light of the QUARTZ trial results [Citation16], which showed a lack of survival benefit adding WBRT to dexamethasone and best supportive care. However, it is worth noting that patients (n = 538) included in QUARTZ trial were not stratified according to RPA class and that 30% of the patients had a solitary brain metastasis. Allocation of patients with 1–3 BM and RPA class 1 or 2 in the 2 above-mentioned arms is in conflict with current guidelines. These patients should be treated with SRS or surgery (surgery for single CNS metastasis) with the addition of systemic treatment where deemed necessary [Citation17]. However, the majority of patients included in the QUARTZ trial had a bad prognosis (37% RPA class 3 and 54% RPA class 2 in the WBRT arm) and were not optimal candidates for local CNS treatment [Citation16].
The aim of this study was to find prognostic factors that can influence OS in BM lung cancer patients treated with WBRT, in order to identify which patients will live long enough to experience the palliative benefit of WBRT, regarding disease control in the CNS [Citation18]
Methods
Patient population
We designed a retrospective cohort study. The patient population consisted of subjects with brain metastasized lung cancer who received palliative WBRT (no patient received adjuvant or prophylactic WBRT) at Karolinska University Hospital from the first of January 2010 until the first of January 2015. We collected demographic data, information about received oncological therapy, radiological evaluation, histopathological results and physician evaluation of performance status before receiving WBRT. Sixteen patients received a radiation dose of 3 Gy ×10 while the rest received 4 Gy ×5. Ethical approval was obtained from the regional ethical review board of Stockholm.
RPA and GPA classification
We divided patients in RPA classes and GPA groups according to age at the time of WBRT, control status of primary tumor, KPS, number of BM and the presence of extracranial disease (). In 85% of the patients brain, MRI with contrast was performed. Rest of the patients (15%) had only CT-verified metastases, but all of these patients had >8 BM, therefore not influencing GPA scoring. The presence of extracranial disease and control status of primary tumor were confirmed with CT scan of the thorax and abdomen in all patients. CT thorax and abdomen was performed in ≤4 weeks from the time of BM diagnosis. We divided patients into three GPA groups, group 0 (0–1 points), group 1 (1.5–2.5 points) and group 2 (3–4 points), where group 0 was the worst prognostic group and group 2 the best prognostic group.
Table 1. RPA and GPA classification.
Statistical analysis
For the characterization of the cohort, the normally distributed variables are presented as mean ± standard deviation (SD), while non-normally distributed variables are presented as median (interquartile range, IQR). The normality of all continuous variables was assessed by skewness. Kaplan–Meier curves were plotted to determine overall survival (OS) from the time of received WBRT (first day/fraction) until death for the whole cohort and by GPA group and RPA class. Patients who were alive at the time of data collection were treated as censored observations during the analysis. Curves were compared with the log-rank test. A subgroup OS analysis was performed stratified by RPA class.
Predictors of OS were identified by Cox regression analyses. A univariate Cox regression analysis was undertaken with gender, age >70 years, histopathology, separate histopathology for adenocarcinoma versus non-adenocarcinoma, CNS metastases at diagnosis, surgery for CNS metastases, symptomatic disease, EGFR status, ALK status, KRAS status, systemic treatment of BM before WBRT, SRS, GPA group and RPA class as independent variables. We used an age cutoff for the whole population where elderly patients were considered those over 70 years (106 patients) and younger ≤70 years (174 patients). We chose this age cutoff taking into consideration existing literature for the treatment of elderly NSCLC patients [Citation19,Citation20], the mean age of CNS diagnosis in our cohort and relevant literature regarding toxicity and efficacy of brain irradiation in elderly patients with primary CNS tumors [Citation21]. The results from the univariate analysis with a p value <.25, as well as variables considered clinically significant guided the selection of variables for the multivariate Cox regression analysis. The non-significant variables were removed from the model by stepwise backward selection. The multivariate analysis was done separately for RPA class and GPA class, due to overlapping. All statistical analyzes were done with SPSS version 23 (IBM Corp, Armonk, NY, USA).
Results
Patient characteristics
Our cohort consisted of 282 patients with BM lung cancer who received WBRT. Two patients were excluded because of missing follow up data and 280 patients were included in the final analysis. Of these patients, five were alive at the time of data collection and were censored, while 275 had died. Fifteen patients died during WBRT, due to disease progression, without receiving the planned dose and were included in our analyses. The demographics are presented in . The mean age (SD) for the whole cohort was 66.6 (10.2), while in the subgroup of patients over 70 years was 76.45 (5.03). Only 30 patients were tested for KRAS mutation status, and therefore, we did not include this parameter in the multivariate analysis. There were relatively few patients (73) tested for ALK-EML4 translocation and of the 7 ALK-positive cases found, three patients did not receive treatment with tyrosine kinase inhibitor (TKI). There were totally 28 patients with EGFR exon 19 deletion or exon 21 L858R point mutation, 25 of which received treatment with TKI. Due to the low representation of patients who did not receive TKI despite the presence of a driver mutation, TKI treatment was not included as a parameter in survival analysis.
Table 2. Baseline demographics and disease characteristics.
Overall survival analyses
The percentage of patients who died 1, 2 and 3 months from start of WBRT was 27.1%, 43.1% and 54.7%, respectively (). OS analyses according to RPA class are shown in and according to GPA groups in . Median OS was 324 (95% CI: 197–451), 130 (95% CI: 94–166) and 41 (95% CI: 33–49) days for RPA class 1, 2 and 3, respectively. Median OS for GPA groups 0, 1 and 2 was 55 (95% CI: 46–64), 166 (95% CI: 109–223) and 110 (95% CI: 0–233) days, respectively. Pairwise log rank testing showed statistically significant differences with p< .0001 between RPA classes 1–3 and 2–3 as well as GPA groups 0–1.
Figure 1. OS whole cohort. Cumulative percent: 21.7% of patients died at the first month, 43.1% at 2 months and 54.7% at 3 months after WBRT.
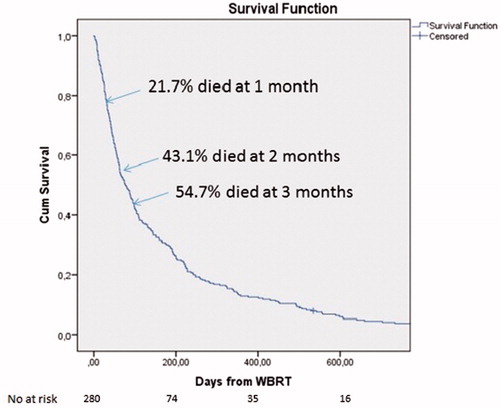
Figure 2. (A) OS analysis for RPA classes, pairwise log rank test has shown statistically significant difference with p < .0001 between RPA classes 1 versus 3 and 2 versus 3. (B) OS analysis for GPA groups, pairwise log rank test has shown statistically significant difference with p < .0001 between GPA groups 0 versus 1. (C) OS according to GPA for RPA class 2 subgroup (165 patients), there was a statistically significant OS difference between GPA group 0 (79 patients) and GPA group 1 (78 patients) with p = .004. (D) OS according to GPA/age for RPA class 2 subgroup, Group A: Age ≤70, GPA group 0 [41/165], group B: Age ≤70, GPA group 1 or 2 [66/165], group C: Age> 70, GPA group 1 or 2 [20/165] and group D: Age >70, GPA group 0 [38/165]. The pairwise log rank test showed a statistically significant difference in OS between groups A versus D (p = .036), as well as B vs D (p < .0001).
![Figure 2. (A) OS analysis for RPA classes, pairwise log rank test has shown statistically significant difference with p < .0001 between RPA classes 1 versus 3 and 2 versus 3. (B) OS analysis for GPA groups, pairwise log rank test has shown statistically significant difference with p < .0001 between GPA groups 0 versus 1. (C) OS according to GPA for RPA class 2 subgroup (165 patients), there was a statistically significant OS difference between GPA group 0 (79 patients) and GPA group 1 (78 patients) with p = .004. (D) OS according to GPA/age for RPA class 2 subgroup, Group A: Age ≤70, GPA group 0 [41/165], group B: Age ≤70, GPA group 1 or 2 [66/165], group C: Age> 70, GPA group 1 or 2 [20/165] and group D: Age >70, GPA group 0 [38/165]. The pairwise log rank test showed a statistically significant difference in OS between groups A versus D (p = .036), as well as B vs D (p < .0001).](/cms/asset/3acae2cb-518a-4a7f-87cd-82e103679c18/ionc_a_1386799_f0002_c.jpg)
A statistically significant longer OS was observed for patients <70 years (p< .0001). A subgroup OS analysis was performed for RPA classes taking into account GPA groups. In RPA class 1, no analysis was feasible because of small patient number (n= 13). In RPA class 3 subgroup, there were only 11 patients in GPA group 1 and 1 patient in GPA group 2 compared to 89 patients in GPA group 0; therefore, no further analysis could be made. In RPA class 2 subgroup (n= 165), there was a statistically significant OS difference between GPA group 0 (79 patients) and GPA group 1 (78 patients) with p= .004 ().
Univariate survival analysis showed that age (both continuous and dichotomous variable with 70 years as cutoff), open surgery in CNS before salvage WBRT, RPA class and GPA class group had a statistically significant impact on OS (). In the subgroup of patients with age over 70 (n = 106), age as continuous variable had no significant impact on OS (p = .294). There were only three patients who received surgery in CNS after WBRT (debulking surgery), and therefore, we did not include these patients in the multivariate analysis. All other patients receiving open CNS surgery had one solitary metastasis at the initial diagnosis of brain dissemination. In the multivariate analysis including RPA class, we found age and RPA class to be independent prognostic factors. In the multivariate analysis including GPA class, the independent prognostic factors were CNS surgery before salvage WBRT, age, symptomatic CNS disease and GPA group ().
Table 3. Univariate Cox regression analysis.
Table 4. Multivariate Cox-regression analyses for OS.
Based on the results from the RPA subgroup OS analysis mentioned earlier, we performed a multivariate Cox regression analysis in the different RPA subgroups. In RPA 1 subgroup, no analysis was made because of the low number of patients (n = 13). In RPA 3 subgroup (n= 101), there were no independent variables found which could influence OS. In RPA class 2 subgroup (n= 165), age, CNS surgery before salvage WBRT and GPA group (1 vs. 0) were found to be statistically significant (). There was not a statistically significant difference found between GPA group 2 versus 0, something which can be explained by the low number of patients in GPA group 2 (n= 8).
In order to further explore the heterogeneity in RPA class 2 subgroup, we performed a Kaplan–Meier OS analysis taking into account the different age and GPA groups (). We divided the combined groups as described below; Group A (‘intermediate group’): age ≤70 and GPA group 0 [41/165], group B (‘good group’): age ≤70 and GPA group 1 or 2 [66/165], group C (‘intermediate group’): age >70 and GPA group 1 or 2 [20/165] and group D (‘bad group’): age >70 and GPA group 0 [38/165]. The pairwise log rank test showed a statistically significant difference in OS between groups A and D, as well as B and D. The median OS in days was 193 [95% CI: 147–239] (B group), 118 [95% CI: 55–181] (A group), 90 [95% CI: 18–162] (C group) and 89 [95% CI: 51–127] (D group). The good prognostic group B and the intermediate prognostic group A (younger patients and bad GPA group) had the best OS, whereas the bad prognostic group and the intermediate with older patients and good GPA score had the worst median OS, 3 months or lower.
Discussion
In the majority of the existing prognostic scoring systems for BM patients receiving cranial irradiation, there are considerable drawbacks, mainly caused by the limited number of patients studied (i.e. 65 patients in SIR publication, 110 patients in BSBM publication) and the heterogeneity regarding the kind of radiotherapy used (WBRT or SRS) [Citation7–12]. The BSBM is a prognostic score for patients who receive stereotactic radiosurgery (SRS) and includes KPS, the presence of extracranial disease and control of primary tumor as parameters [Citation11]. The modified BSBM predicts neurological outcomes, independently of life expectancy, in SRS-treated patients with brain metastases [Citation12]. The Score Index for Radiosurgery for BMs (SIR) takes into consideration the number and volume of BMs [Citation10], therefore requiring extra input from neuro-radiologists making it more difficult to apply in daily clinical praxis. In this real life cohort study, RPA and disease-specific GPA were used, since they are the most practical, most validated and most used scoring systems for patients with BM solid tumors [Citation15].
RPA class 1 patients have a better prognosis with a median OS often exceeding 7 months and should therefore receive local CNS treatment if indicated, whereas RPA class 3 patients have the worst prognosis, rarely exceeding 2 months and should not receive WBRT or other local CNS treatment [Citation6,Citation13]. RPA classification has received criticism since it depends mostly on PS [Citation7,Citation22]. Lutterbach et al divided RPA class 3 into three subgroups, according to status of the primary tumor, number of brain metastases and age, showing that the RPA class 3a group had longer survival (3.2 months) compared to class 3b (1.9 months) and 3c (1.2 months) and suggested that patients in group 3a could be considered for local CNS treatment [Citation22]. In this study there were only 51 patients in RPA class 3a (of totally 408 patients in RPA class 3) with median age 55 years, of which 59% had surgically resected single metastasis, something which may indicate bias [Citation22]. In our cohort median OS for RPA class 3 was 41 days [Citation12] (95% CI 35–49 days), something which supports the omission of WBRT in this patient group. In the heterogeneous RPA 2 subgroup of our cohort, there were 79 patients who were classified in the GPA group 0 and 78 in the GPA group 1. It is of major clinical interest to find a way to identify which RPA 2 patients should receive WBRT. WBRT has a palliative effect regarding CNS disease control, but not any proven OS benefit. Therefore it is important to identify which patients will live long enough to overcome the early toxicity related to WBRT and experience its palliative benefit [Citation18,Citation23].
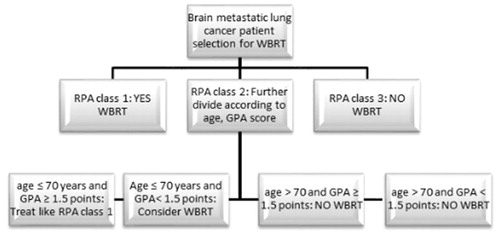
In this real life cohort, we found that age over 70 years is an independent prognostic factor for OS after WBRT. When taken as continuous variable, higher age had a negative impact on OS in the whole cohort, whereas no such impact was seen in the subgroup of patients over 70 years. In this subgroup of patients over 70 years, age was normally distributed with a mean of 76.45 (±5.03). We also found that age over 70 years and GPA score can help choosing the right patient to receive WBRT in the heterogeneous RPA 2 subgroup in a two-step procedure (). In the intermediate prognostic groups of RPA 2 patients, with either age ≤70 years and GPA <1.5 points, or age >70 and GPA ≥1.5 points, age seems to be a stronger prognostic factor for OS compared to GPA.
Figure 3. Diagram for the selection of brain metastasized lung cancer patients who should receive WBRT.
The median OS found in RPA classes and GPA groups in our cohort are similar to those reported in the literature [Citation6,Citation13–15]. The exception was GPA group 2 (GPA score 3–4) where we found a slightly worse OS than group 1. This may be explained by the low number of patients in this group (n= 13), of which three had primary CNS metastasized disease and died unexpectedly a few days after the completion of WBRT without receiving any other oncological treatment. GPA group 2 patients have generally a better prognosis than GPA group 1 [Citation13–15].
Surgery for BM before WBRT was an independent prognostic factor for OS in the multivariate analysis both for RPA class 2 subgroup and GPA. Selection bias is a possible explanation for this finding, as there were different time periods between CNS surgery and WBRT in these patients, it was mostly younger patients with good PS and solitary brain metastasis who were fit for surgery, and there was a small number of patients who underwent surgery in our cohort. Surgery can be a reasonable treatment option for selected cases of NSCLC patients who present with single BM [Citation24,Citation25]
Symptomatic CNS disease was an independent prognostic factor for poor OS in the GPA multivariate analysis. This finding has a reasonable clinical explanation, since patients who present with asymptomatic disease generally have lower disease burden in the CNS and less aggressive tumors. In daily clinical praxis, almost all patients with brain metastasized solid tumors present with CNS symptoms. In our cohort, 93.2% of the patients had symptoms at diagnosis.
In a recently published study from Japan on brain metastasized NSCLC treated with WBRT adenocarcinoma histology was an independent prognostic factor for OS [Citation26], something which was not confirmed in our cohort. This may be explained by the higher frequency of EGFR-mutated lung adenocarcinomas in Asian compared to Caucasian patients [Citation27], taking into account that EGFR-mutated patients with CNS metastases have a better prognosis, due to the existing TKI therapies that are effective in the CNS [Citation28,Citation29].
In general, upfront TKI treatment should be preferred in BM lung adenocarcinoma patients with oncogenic-driven tumors, taking into account the intracranial efficacy and the absence of CNS toxicity [Citation23]. A recent retrospective analysis compared SRS upfront followed by TKI, WBRT upfront followed by TKI or TKI upfront followed by WBRT or SRS at intracranial progression for brain metastatic NSCLC patients who were TKI naive. The SRS upfront arm had a significant prolongation of OS compared to the two other groups (46, 30 and 25 months, respectively) [Citation30]. The results of this trial should be interpreted with caution. It was a retrospective analysis prone to selection bias, no report on acute/late CNS toxicity from SRS was reported, there was a certain heterogeneity regarding prognostic factors among treatment arms, and TKI in the SRS or WBRT upfront groups was also given upfront (directly after the completion of radiotherapy). This result needs to be verified in a prospective trial, especially since WBRT or SRS or combination of both has not proven to have any OS benefit in almost all reported trials, the majority of NSCLC patients with BM die from extracranial disease progression, and Osimertinib upon disease progression (recently approved standard therapy, not given in this trial) has a remarkable CNS activity [Citation23,Citation31]. Furthermore, FLAURA trial presented at ESMO, 2017, showed that Osimertinib in the first line (upfront) is more effective than first and second generation TKIs, especially for patients with BM [Citation32]
Only 16 patients received a WBRT dose of 3 Gy ×10, while 264 patients received 4 Gy ×5. A comparison between these two fractionation schedules is not meaningful due to discrepancy between the number of patients receiving different schedules, but mostly due to existing literature that has not shown any difference in efficacy with different WBRT fractionations [Citation17,Citation18].
The major limitations of our study are its retrospective nature and the small number of patients in some subgroup analyses, making it prone to selection bias. However, we have observed a large number of patients who died early after the completion of WBRT, something which constitutes the selection of ‘healthier’ patients less likely. On the other hand, it is really important to include all patient categories (all comers) in such analyses, in order to identify all possible prognostic factors. The majority of the subgroup analyses done in our study had a relatively large number of patients, and in the cases where the number was low, no conclusion can be drown. The recommendations from our study results regarding the correct use of WBRT is applicable only in patients who benefit most from WBRT. As mentioned above, the vast majority of oncogenic driven tumors should be treated with TKI upfront, and patients with good prognostic factors and 1–3 CNS metastases with surgery or SRS [Citation17,Citation23].
Conclusions
From the results of the present study, we suggest that the selection of brain metastatic lung cancer patients who should receive WBRT, if clinically indicated, could be done in two steps. RPA classification is a useful and practical prognostic tool for making a first step assessment, omitting WBRT for RPA class 3 patients without further evaluation. RPA class 1 should receive WBRT, if clinically indicated. The second-step assessment is solely for RPA class 2 patients, where we suggest that patients with age ≤70 years and GPA ≥1.5 points should be treated as RPA 1 patients. WBRT should be omitted in RPA 2 patients with age >70. In RPA 2 patients with age ≤70 years and GPA <1.5 points WBRT could be a reasonable option (). This real life cohort study showed that WBRT should be considered in a larger proportion of BM NSCLC patients compared to QUARTZ trial results, where all patients older than 65 years (not RPA class 1) do not seem to derive any benefit from WBRT [Citation16].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. JCO. 2005;23:6207–6219.
- Taimur S, Edelman MJ. Treatment options for brain metastases in patients with non-small-cell lung cancer. Curr Oncol Rep. 2003;5:342–346.
- Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008;72:1311–1318.
- Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70.
- DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796.
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751.
- Rades D, Dunst J, Schild SE. A new scoring system to predicting the survival of patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol. 2008;184:251.
- Rades D, Dziggel L, Haatanen T, et al. Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys. 2011;80:1122–1127.
- Rades D, Dziggel L, Nagy V, et al. A new survival score for patients with brain metastases who received whole-brain radiotherapy (WBRT) alone. Radiother Oncol. 2013;108:123–127.
- Weltman E, Salvajoli JV, Brandt RA, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155–1161.
- Lorenzoni J, Devriendt D, Massager N, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–224.
- Serizawa T, Higuchi Y, Nagano O, et al. A new grading system focusing on neurological outcomes for brain metastases treated with stereotactic radiosurgery: the modified Basic Score for Brain Metastases. J Neurosurg. 2014;121:35–43.
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514.
- Villa S, Weber DC, Moretones C, et al. Validation of the new Graded Prognostic Assessment scale for brain metastases: a multicenter prospective study. Radiat Oncol. 2011;6:23.
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. JCO. 2012;30:419–425.
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014.
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v1–v27.
- Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4:CD003869.
- Blanco R, Maestu I, de la Torre MG, et al. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. 2015;26:451–463.
- Gajra A, Jatoi A. Non-small-cell lung cancer in elderly patients: a discussion of treatment options. JCO. 2014;32:2562–2569.
- Sulman EP, Ismaila N, Armstrong TS, et al. Radiation therapy for glioblastoma: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Guideline. JCO. 2017;35:361–369.
- Lutterbach J, Bartelt S, Stancu E, et al. Patients with brain metastases: hope for recursive partitioning analysis (RPA) class 3. Radiother Oncol. 2002;63:339–345.
- Tsakonas G, De Petris L, Ekman S. Management of brain metastasized non-small cell lung cancer (NSCLC) – From local treatment to new systemic therapies. Cancer Treat Rev. 2017;54:122–131.
- Granone P, Margaritora S, D'Andrilli A, et al. Non-small cell lung cancer with single brain metastasis: the role of surgical treatment. Eur J Cardio-Thorac Surg. 2001;20:361–366.
- Putora PM, Ess S, Panje C, et al. Prognostic significance of histology after resection of brain metastases and whole brain radiotherapy in non-small cell lung cancer (NSCLC). Clin Exp Metastasis. 2015;32:143–149.
- Harada H, Asakura H, Ogawa H, et al. Prognostic factors in patients with brain metastasis from non-small cell lung cancer treated with whole-brain radiotherapy. J Can Res Ther. 2016;12:267–270.
- Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5:2892–2911.
- Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–4414.
- Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11:380–390.
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. JCO. 2017;35:1070–1077.
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395.
- Ramalingam S, Reungwetwattana T, Chewaskulyong B, et al. Osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFRm advanced NSCLC: FLAURA. ESMO, Madrid; 2017.
