Abstract
Background: Oropharyngeal carcinomas (OPCs) constitute a significant and increasing proportion of head and neck carcinomas and are an important global cause of morbidity and mortality. The purpose of this study was to determine trends in incidence and survival in OPC in the Danish population from 1980 to 2014.
Methods: This study included all patients registered in the nationwide Danish Cancer Registry over the period 1980–2014. The age-adjusted incidence rates (AAIR) per 100,000, annual percentage change (APC) and average annual percent change (AAPC) were evaluated. Five-year relative survival (RS) was calculated with Cox regression analyses in relation to gender, anatomical location and histology.
Results: A total of 6555 patients (69% male) were included, with a median age at diagnosis of 60 years. The AAIR of patients with OPC increased from 0.815 per 100,000 in 1980 to 4.51 per 100,000 in 2014 with an AAPC of 5.3. The 5-year RS increased significantly from 33.1% over the period 1980–1984 to 58.5% (25.4% points) over the period 2010–2014. With no significant difference stratified for gender. Tumors located at the palatine tonsils (n = 3333) and salivary gland OPC (n = 90) had significantly better survival compared with other sub-locations and histology subtypes. In the APC model the birth cohort effect rate ratio increased until 1925 and then decreased until 1935 from which point it increased in the last cohorts.
Conclusions: In this population-based study, we observed a significant increase in the incidence of OPCs and in the RS for OPC. We also identified a profound birth cohort effect on the incidence.
Introduction
The annual, global incidence of pharyngeal cancers is approximately 230,000, accounting for more than 1.5% of all cancers worldwide, and resulting in nearly 150,000 deaths yearly [Citation1].
The incidence rates for oropharyngeal cancers (OPC) have been increasing during recent decades, predominantly in developed countries and in younger patients (<60 years) [Citation2], with the highest incidence observed in northern Europe [Citation3]. The increasing incidence is recognized as a result of the growing subset of OPCs associated with human papillomavirus (HPV) infection, whereas the incidence of HPV-negative OPCs has been reported to be stable or declining [Citation2–5]. Patients with HPV-associated OPC are known to have longer overall and disease-free survival compared with HPV-negative patients [Citation6]. Worldwide tobacco and alcohol use remain the most important risk factors for OPC [Citation7].
In more than 90% of cases pharyngeal cancers consist of squamous cell carcinomas (SCC) [Citation7], and knowledge regarding the incidence and survival of the remaining histological subtypes is very sparse. Furthermore, birth cohort-effects on incidence of OPC are rarely analyzed. The aims of this study were to evaluate the survival and incidence trends based on gender, histology and tumor localization of patients diagnosed with OPC in the Danish population during the period 1980–2014, and to evaluate a possible cohort-effect on the incidence.
Material and methods
Data included in this study are derived from the Danish Cancer Registry (DCR), which contains data on all cancers diagnosed in Denmark from the year 1943 [Citation8] and onwards. Reporting to the DCR became mandatory in 1987. The DCR is linked to the central population register (CPR) in Denmark, which since 1968 has provided every resident in Denmark with a unique personal identification number, to which information regarding vital status and emigration is linked [Citation9]. All the patients registered with OPC in the DCR between 1980 and 2014 were included in the study. From 1978 all patients reported to the DCR was coded using ICD-O-1, when the DCR became digitalized in 2004, all patients from 1978 to this point were then converted ICD-10 via ICD-O-2 and ICD-O3 according to international guidelines [Citation8]. The ICD10-codes included in this study were DC01, DC02.4, DC05.1, DC05.2, DC09.0, DC09.1, DC09.2, DC09.8, DC09.9, DC10.2, DC10.3, DC10.8 and DC10.9. The patients were divided into four groups: palatine tonsils (i.e., DC09.0, DC09.1, DC09.2, DC09.8, DC09.9), walls of oropharynx (i.e., DC10.2, DC10.3, DC10.8, DC10.9), base of tongue (i.e., DC01, DC02.4) and others (i.e., DC05.1, DC05.2, DC10.0). The histological information on tumors was derived from the ICD-O-3 classification via the MORPHO3 registration from the DCR [Citation10]. Based on the 56 different histology subtypes identified, we divided patients into the following four groups: SCC, adenocarcinomas, salivary gland tumors and others. With the introduction of the ICD-10 haematolymphoid tumors are no longer classified by their anatomical location, but instead by their histology type, therefore, it was not possible to include these tumors located in the oropharynx based on the ICD10-codes. For this reason these tumors were excluded.
In this study the incidence rates were calculated as age-adjusted incidence rates (AAIR), this method circumvents the fact that most rates, including incidence of cancer, is age dependent by adjusting the incidence to the distribution of the age groups in the given population, so a high incidence is not merely a result of a population with a large amount of elderly people or vice versa. This allows the numbers to be compared with any given population directly. The survival was calculated as the relative survival (RS), which is the survival of the patients with OPC relative to the survival of the rest of the population. This method ensures that any observed change in survival among patients with OPC is not merely a change in the survival of the general population, but is also the reason why the graphs of the survival are not necessarily always declining because as the group becomes smaller, the few patients who are left could have better survival relative to the general population. Finally we calculated an age-period-cohort (APC) model, where the effect of age, period (i.e., the year of diagnosis), and the cohort (i.e., the year of birth) on incidence of OPC was evaluated.
Data regarding the age at diagnosis were derived from the DCR, and vital status and emigration were obtained from the CPR.
We did not include information on tumor, node and metastasis (TNM) classification because this information was not part of the DCR before 2004, and the data was not of high quality even after 2004.
Statistical analysis
Statistical analyses were performed in R statistics version 3.3.3 (Stanford University, Stanford, CA, USA) [Citation11]. Incidence rates per 100.000 were calculated using the direct method with the EpiTools package [Citation12] using the WHO world standard population age-group distribution and the Danish population as reference [Citation13]. The average annual percent change (AAPC) was calculated using Joinpoint trend analysis software v. 4.2.0.2, with growth assumed to be logarithmic with the formula ln(y) = xb. The Joinpoint regression analysis estimates possible joinpoints (trend breaks), which are significant changes in trends, as previously described [Citation14].
The RS rate was calculated as the ratio of the observed survival rate to the expected survival rate in Denmark matched by age, sex and calendar year with the R package relsurv [Citation15]. The RS is defined as the all-cause observed survival in the cancer population in the study divided by the expected survival of a comparable group in the general population. The RS is, therefore, a measure of the excess mortality associated with a diagnosis of OPC. The expected survival was estimated using the Ederer II method [Citation16]. Patients who were alive at the last date of follow-up were censored at this date. The date for the last follow-up was 1st of December 2016.
The APC model was calculated with the R package Epi [Citation17]. The reference year was for cohort effect 1900 and for period effect 1982.5. The age and periods were arranged in five year intervals. To avoid statistical instability, the analysis was restricted to persons aged between 30 and 84 years.
Results
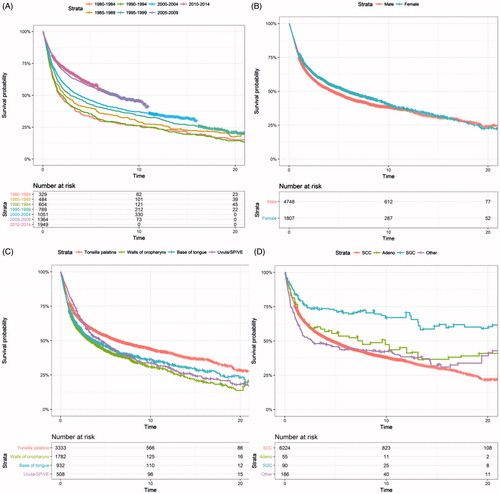
From the DCR, 6555 patients with OPC were included. The patients were predominantly male [4748 men (69%)] (). The median age at diagnosis was 60 years. The most common anatomical location was the palatine tonsils, accounting for 49%, and the most common histology was squamous cell carcinoma, accounting for 91% ().
Table 1. Univariate and multivariate Cox regression analyses of patients with OPC in Denmark between 1980–2014.
Incidence
For all patients diagnosed with OPC, the AAIR per 100.000 increased from 0.815 in 1980 to 4.51 in 2014 (p < .05, ), corresponding to a total number of 56 and 399 patients per year (). We observed one significant trend break in 1986, when the annual percent change (APC) decreased from 8.50 to 4.65%. The AAPC was 5.3% ().
Figure 1. Incidence rates and number of cases per year for A and B: all OPCs, C: men, D: women, E: palatine tonsils and F: squamous cell carcinoma.
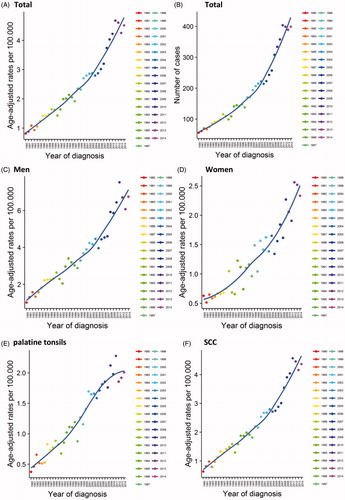
Table 2. Joinpoint regression analysis demonstrating trends in AAIR.
For males the AAIR per 100.000 increased from 1.02 in 1980 to 6.75 in 2014, with an AAPC of 5.6% (, ). For females the AAIR per 100.000 increased from 0.628 in 1980 to 2.33 in 2014, with an AAPC of 4.6% (, ).
Considering the anatomical location, the AAIR per 100,000 of cancer located in the palatine tonsils (), the walls of oropharynx, and base of the tongue, all increased continuously throughout the study period, whereas cancer located in the uvula, soft palate and vallecula epiglottica showed an overall increase, but with a significant trend break with a decrease after 2001 ().
Squamous cell carcinoma () and adenocarcinoma showed a significant increase in the AAIR per 100,000. The remaining histological subtypes showed no significant change in incidence.
The reporting to the DCR became mandatory in 1987. We, therefore, analyzed the incidence rates for the period 1987–2014 as well as for 1980–2014. These analyses showed no significant difference (Table S1).
Relative survival
Patients were followed-up for a median period of 2.6 years (range 0–35.2 years)
The RS at 5 years increased significantly from 33.1% (95%CI: 27.9; 39.2) in 1980–1984 to 58.5% (95%CI: 55.4; 61.8) in 2010–2014. We examined factors associated with better RS () and found that the palatine tonsils had a significantly better survival than other locations. Concerning the different histological types, carcinomas of the salivary glands had significantly better prognosis than SCC [, ]. There were no significant differences between the survival of men and women [, ].
APC model
For all OPCs the highest incidence was observed between the ages of 55 and 60 years. The age at which the highest incidence was observed was not different between the sexes ().
Figure 3. Age-period-cohort model for all OPCs, for men and for women. LCI: lower confidence interval; UCI: upper confidence interval; RR: rate ratio.
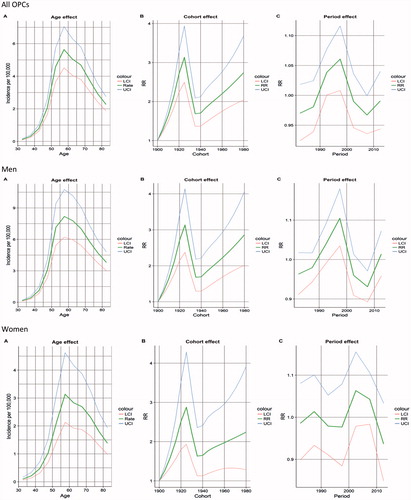
Regarding the cohort effect, the rate ratio increased from the first cohort, reaching the highest in 1925 and then decreasing before 1940 and then increasing again in the remaining cohorts. When stratifying based on gender, this pattern was similar ().
In the period effect, there was a significant increase of the rate ratio up until before the year of 2000, followed by a significant decrease for all OPCs. These changes were only significant for men ().
Discussion
In this large population-based study including 6555 patients, we report that both the incidence and the survival of patients with OPC in Denmark increased during the past 34 years. The increasing incidence observed is similar to reports from several Western countries [Citation18] and has been attributed to changes in sexual behaviors in more recent birth cohorts, including a higher number of sexual partners, resulting in an increased risk of infection with HPV [Citation5,Citation19].
The increasing incidence of OPC, especially for men, raises the question of if offering boys prophylactic HPV vaccines should be introduced as a prevention strategy. Studies show that at moderate to high vaccination rates for girls, the gain of vaccinating boys is limited, due to herd immunity [Citation20]. Therefore, the most economically effective way to decrease HPV-infection is by convincing girls of the benefits of the vaccine. However, the annual report of vaccination status in Denmark from 2016 shows that only 15% of the girls born in 2003 have followed the full HPV vaccination program [Citation21]. Therefore, it appears necessary to include boys to effectively ensure a decrease in HPV positive OPCs.
We found that the RS is increasing. It is well-known that patients with HPV-positive OPC have better survival and a lower risk of recurrence than HPV-negative OPC [Citation6]. Given that the proportion of OPCs that are HPV-positive has been increasing [Citation4] this finding is probably one explanation for the increase in survival. Other explanations include improvements in radiation therapy techniques [Citation22], the recent development of less invasive surgical procedures such as transoral robotic surgery [Citation23], and a fast-track program introduced in 2007 to optimize the diagnosis and treatment for patients suspected of head and neck cancer. Finally, national guidelines for the evaluation and treatment for head and neck carcinomas including OPC were introduced in 2011.
The salivary gland OPC group was small (n = 90) and, therefore, results of this group should be interpreted carefully, however, the better survival of this group was statistical significant and is worth noting.
We identified an interesting cohort effect. One explanation we find plausible is that the large decrease in OPC in citizens born in 1925 and the following 10 years might be due to effects of the Second World War, occurring when these cohorts reached a sexually active age. The war might have resulted in a less promiscuous lifestyle among these cohorts, simply because the curfews and obligatory blackouts resulted in less opportunity to meet new sex partners and in a more restricted lifestyle in general. A Korean study showed a similar cohort effect and attributed this to a change in smoking behavior [Citation24]. This cause could also be the case in Denmark as the supply of tobacco was limited during the war. The increase from the 1940 cohort could be due to the 1960s’ youth rebellions and the introduction of the contraceptive pill, leading to a more promiscuous lifestyle among these cohorts. We emphasize that these theories are purely speculative.
The TNM classification is an important factor associated with the survival of the patients, and a limitation of our study is that this classification was not included. However, given that the study covers 34 years, there would likely have been a great risk of stage migration; either due to new editions of the guidelines for the TNM classification, or because of patients expectedly would be diagnosed in earlier stages in the latter years as a result of improved and more effective healthcare service. Another limitation of this study is the lack of tumor HPV- or p16-status was not included because we did not have access to this information.
There remains a lack of consensus on the borders of the pharynx among previously published studies, and disease codes that refer to more than one division of the pharynx are often included in studies that examine a specific division. This lack of consensus makes the applicability of former studies disputable and the comparison of results problematic. Therefore, a large focus of this study was to include only those ICD-10 codes that are strictly referring to oropharyngeal cancer.
We believe that our study adds significant new knowledge to a previous similar study performed by Fakhry et al. [Citation25]. The statistical analysis of the latter study was limited and only focused on an increase in the number of survivors. This approach was an interesting perspective. However, in contrast to our study, location, histology and gender in the statistical analysis were not explored. Furthermore, the ICD-10 codes included in the study by Fakhry et al. were not specifically related to the oropharynx. DC14.0, DC14.2 and DC14.8 referring to cancer of pharynx UNS, cancer of Waldeyer ring and cancer of overlapping sites of lip, oral cavity and pharynx, respectively, were all included. These sites consist partially of OPC but not exclusively, resulting in a significant portion of the data being derived from patients with non-OPC.
In the present study, using data from the DCR, we identified more than 6000 patients with OPC over the period from 1980 to 2014. We report that both the incidence and survival increased for patients with OPC over the complete period of the study, and we have no reason to believe that there will be fewer patients with OPC in the years to come. Furthermore, we report a profound cohort effect explaining trend changes.
IONC_A_1390251_Supplementary_Information.docx
Download MS Word (12.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559.
- Carlander A-LF, Grønhøj Larsen C, Jensen DH, et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: a Danish population-based study from 2011 to 2014. Eur J Cancer. 2017;70:75–82.
- Garnaes E, Kiss K, Andersen L, et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000–2010: the largest registry-based study to date. Int J Cancer. 2015;136:2196–2203.
- Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;28:3269–3272.
- Grønhøj LC, Jensen DH, Carlander A-LF, et al. Novel nomograms for survival and progression in HPV + and HPV– oropharyngeal cancer: a population-based study of 1,542 consecutive patients. Oncotarget. 2016;7:71761–71772.
- Lambert R, Sauvaget C, de Camargo Cancela M, et al. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633–641.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45.
- Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39:12–16.
- Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry-history, content, quality and use. Dan Med Bull. 1997;44:535–539.
- Team RC. R: A language and environment for statistical computing; 2017 [cited 2017 June 27]. Available from: https://www.r-project.org/.
- Aragon TJ. Epitools: epidemiology Tools. R package version 0.5-9; 2017 [cited 2017 June 26]. Available from: https://cran.r-project.org/package=epitools.
- Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age standardization of rates: a new who standard; 2017 [cited 2017 Sept 12]. Available from: http://www.who.int/healthinfo/paper31.pdf.
- Karnov K, Grønhøj Larsen C, Jensen D, et al. Increasing incidence and survival in oral cancer: a nationwide Danish study from 1980 to 2014. Acta Oncol (Madr). 2017;56:1204–1209.
- Perme MP. Relsurv: Relative survival. R package version 2.0-9; 2016 [cited 2017 June 26]. Available from: https://cran.r-project.org/package=relsurv.
- Ederer FM, Axtell L, Cutler SJ. The relative survival rate: a statistical methodology. 2017; p. 101–121 [cited 2017 June 26]. Available from: http://www.pauldickman.com/survival/handouts/41-Therelativesurvivalrate-astatisticalmethodology.pdf.
- Bendix C, Martyn P, Esa Laara MH. Epi: a package for statistical analysis in epidemiology. R package version 2.15; 2017 [cited 2017 June 26]. Available from: https://cran.r-project.org/package=Epi.
- Hammarstedt L, Dahlstrand H, Lindquist D, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988–992.
- Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623.
- Brisson M, Van De Velde N, Franco EL, et al. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis. 2011;204:372–376.
- Sundhedsstyrelsen. Børnevaccinationsprogrammet Årsrapport 2016. [Childvaccination-program Year-report 2016] Copenhagen; 2017 [cited 2017 May 22]. Available from: https://www.sst.dk/da/udgivelser/2017/∼/media/C18E03415F2044D1854DFD7761D1C7E8.ashx.
- May JT, Rao N, Sabater RD, et al. Intensity-modulated radiation therapy as primary treatment for oropharyngeal squamous cell carcinoma. Head Neck. 2013;35:1796–1800.
- Channir HI, Rubek N, Nielsen HU, et al. Transoral robotic surgery for the management of head and neck squamous cell carcinoma of unknown primary. Acta Otolaryngol. 2015;135:1051–1057.
- Jee Y, Shin A, Lee J-K, et al. Decreases in smoking-related cancer mortality rates are associated with birth cohort effects in Korean men. Int J Environ Res Public Health. 2016;13:1208.
- Fakhry C, Andersen KK, Eisele DW, et al. Oropharyngeal cancer survivorship in Denmark, 1977-2012. Oral Oncol. 2015;51:982–984.