Abstract
Background: Treatment for oropharyngeal squamous cell carcinoma (OPSCC) has changed, as the proportion of human papilloma virus (HPV)-related disease has increased. We evaluated nationwide information on its management and outcome during the treatment paradigm change period.
Methods: We included all patients diagnosed and treated for OPSCC at the five Finnish university hospitals from 2000 to 2009. Patient records and pathology registries provided the clinicopathological data. p16 staining was performed on primary tumor samples of patients who had received treatment with curative intent.
Results: A total of 674 patients were diagnosed and treated for OPSCC and the incidence increased along the study period. Of the evaluable tumors 58.5% were p16-positive and the number of p16-positive tumors increased along the years. The treatment was given with curative intent for 600 patients and it was completed in 564. Of them, 47.9% underwent primary surgery and 52.1% received definitive oncological treatment. Also, the treatment protocol changed towards a more oncological approach. Among patients treated with curative intent the five-year overall, disease-specific and disease-free survival rates were 60.1, 71.5 and 57.0%. In multivariate analysis, p16-positivity seemed to relate to reduced disease mortality in lateral and anterior-wall disease. Depending on primary tumor localization, also sex, classes T3–4, presence of regional metastasis and radiotherapy modality had an association with disease mortality.
Conclusion: The incidence of p16-positive OPSCC and delivery of definitive oncological treatment increased in Finland during the study period. An improved survival outcome compared with the previous nationwide investigation was observed in this subset of patients.
Introduction
The incidence of oropharyngeal squamous cell carcinoma (OPSCC) has increased during the last decades [Citation1–4]. More precisely, the incidence rates for palatine tonsil (PT) and base of tongue (BOT) squamous cell carcinoma (SCC) have increased [Citation1], and oncogenic human papilloma virus (HPV) infection is likely responsible for this trend [Citation5]. Due to differences in cancer biology, the HPV-associated form of OPSCC is considered to be a distinct disease entity, whereas the HPV-negative OPSCC, with a declining incidence, is biologically more closely associated to other non-HPV-associated head and neck squamous cell carcinomas (HNSCCs) [Citation6]. In the carcinogenesis of HPV-associated OPSCC, the tumor suppressor protein, retinoblastoma, is inactivated. This leads to tumor suppressor protein p16 overexpression [Citation7], which is used as an indirect surrogate marker for HPV association in OPSCC [Citation8]. HPV-positive OPSCC, which has survival rates even 50% higher than its virus-negative counterparts [Citation9], tends to present at a low primary tumor (T) class, but with a more advanced involvement of regional lymph nodes (N) class resulting in a more advanced stage at diagnosis [Citation10–12].
There is a lack of consensus regarding the optimal management of OPSCC, as the outcome results after both definitive oncological and combined treatment have been shown to be relatively similar [Citation10,Citation13–16] The extent of treatment is typically adjusted according to factors such as disease stage, patients’ general condition and comorbidity [Citation17,Citation18]. Modifications of the treatment protocol, according to HPV status, should still be experimental [Citation17–19], although the eighth edition of UICC TNM classification characterizes OPSCC into two distinct subgroups depending on the HPV association of the tumor [Citation20].
Treatment planning warrants new perspectives, as HPV-positive OPSCC is suggested to have better survival regardless of treatment modality [Citation10,Citation11]. According to studies by Ang et al. [Citation5,Citation10], HPV status (or p16 status), smoking history and T and N classes can be used to stratify OPSCC patients into three groups with characteristic overall survival rates. In addition, comorbidity may also be used to stratify OPSCC patients into three groups having distinct survival rates [Citation21]. Several randomized studies evaluating the OPSCC treatment, especially in smaller tumors, are ongoing [Citation22].
In the history, both primary oncological and primary surgical approach have been considered as optimal treatment modalities for OPSCC and the protocol shifts have been attributed to the advances in technology [Citation23]. For some decades, at many centers, surgery used to have a more significant role in the treatment of OPSCC. Later on, delivery of definitive chemoradiotherapy has been increasing, reflecting an aim at better functional outcome as majority of patients remain without surgical intervention [Citation24,Citation25]. However, surgery in treatment of a selected OPSCC patient group may also be beneficial and the rate of surgeries may be currently increasing [Citation23].
Head and neck cancer management is centralized to the five university hospitals in Finland, with a population of 5.5 million people. The Finnish Head and Neck Oncology Working Group maintains national treatment guidelines for these malignancies. The objective of this nationwide Finnish multicenter study is to describe the given treatments and patient outcome in an unselected series of OPSCC patients during the treatment paradigm change period over a 10-year period.
Material and methods
Patients
Our retrospective study population consisted of all patients with an OPSCC diagnosed and treated at one of the five Finnish university hospitals between January 1 2000 and December 31 2009. Only patients with an invasive squamous cell carcinoma (SCC) or its subtype were included. Data collection and p16 staining were retrospectively carried out.
Patient records and pathology registries provided details on age, sex, tumor site, histology, grade of differentiation and TNM classification (UICC seventh Edition) [Citation26], stage, intent of treatment, details on treatment (surgical treatment [Sx], radiotherapy [RT] chemotherapy, [CT] and chemoradiotherapy [CRT]), modality of RT (intensity-modulated radiation therapy (IMRT) or 3D-conformal), tumor recurrence, treatment of recurrent disease and status at last follow up. The tumor sites were analyzed separately for the lateral wall (palatine tonsils, tonsillar fossa and tonsillar pillars), anterior wall (base of tongue and vallecula), superior wall (soft palate and uvula) and posterior wall. We also included patients with subsequent follow up or postoperative RT or CRT given at other hospitals. In these cases, postoperative oncological treatment was carried out according to the same national guidelines. The dates and causes of death were provided by Statistics Finland. A combination treatment (Sx + RT or CRT) was classified as complete if surgery was followed by postoperative RT of at least 45 Gy and definitive RT or CRT were classified as complete if patients had at least 60 Gy of RT. CT was recorded if at least one cycle was implemented. Of all patients who received treatment with curative intent 99 and 75% had a minimum of three- and five-year follow-up or until death. This study was approved by the institutional Research Ethics Board (record number: 179/13/03/02/2013) and a study permission was granted.
Immunohistochemistry
Formalin fixed paraffin blocks were collected from the pathology archives of each hospital. Among the patients with curative intent of treatment, 431 (71.8%) had their tumor block available for p16 immunohistochemistry. The tumor was regarded as p16 positive if more than 70% of tumor cells were strongly immunopositive.
Statistical analysis
SPSS Version 20.0 (SPSS, Inc., Chicago, IL, USA) was used in the statistical analyses. The chi-square test with asymptotic or exact p value explored the statistical associations of categorical variables. Independent samples t-test was used for continuous variables, and normal distribution was observed from histogram. For survival analysis, we used the three- and five-year overall survival (OS), disease-specific survival (DSS), disease-free survival (DFS) and recurrence-free survival (RFS) in the Kaplan-Meier (KM) estimate. The statistical test of survival analysis was the log rank test. The maximum length of follow-up was adjusted to five years to minimize the possibility of follow-up bias. Length of follow-up was calculated from the last day of treatment to the end of follow-up, death of any cause (OS) or death of disease (DSS). DFS was calculated from the last treatment day to the detection of cancer recurrence at any site (primary, neck or distant) or death of any cause. In RFS, only cancer recurrence was considered an endpoint, while other events were censored. The Cox proportional hazards model served in multivariate analysis. The proportional hazards assumption was tested with KM curves. Clinically relevant variables were selected into a manual backward stepwise multivariate analysis. Variables with a p value less than .1 remained in the final step. A double-sided p value less than .05 was considered statistically significant.
Results
Patient population
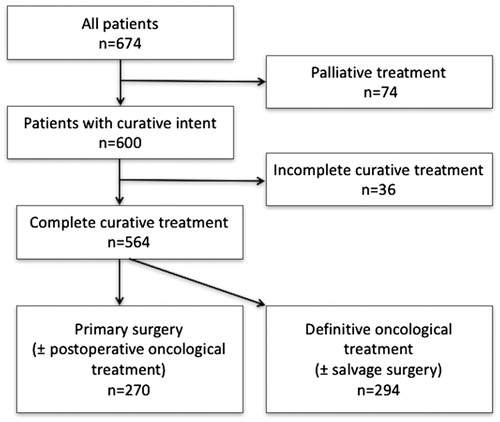
A total of 674 patients with an invasive OPSCC or its histologic variant were identified (). There were 500 (74.2%) males and 174 (25.8%) females, with the mean age of 58.5 years (range, 26.5–90.8). Sixty-one (9.1%) of them had a histological variant of OPSCC (lymphoepithelial SCC, basaloid SCC, adenosquamous SCC, papillar SCC or verrucous SCC). The treatment was intended as curative for 600 (89.0%) and palliative for 74 (11.0%) patients. Treatment with curative intent remained incomplete for 36 patients and thus 564 patients obtained the planned treatment. During the study period, the annual number of OPSCC patients increased. During the years 2000–2004, 260 new OPSCC patients were diagnosed, whereas during 2005–2009 the corresponding figure were 414.
Patients having treatment with curative intent
shows the baseline clinical characteristics of the 600 patients who underwent treatment with curative intent in relation to p16 status, to the main treatment (Sx ± [C]RT or definitive [C]RT ± salvage Sx) and to the treatment years (2000–2004 or 2005–2009). All patients went through magnetic resonance imaging and/or a computer tomography scan as a diagnostic procedure. Most tumors arose from the lateral wall of the oropharynx (64.5%). Of the lateral wall tumors from which p16 evaluation was available, most (68.4%) were p16 positive, which is contrary to the corresponding figures for superior (12.1%) and posterior wall tumors (25.0%). The percentage of p16-positive and negative anterior wall tumors was almost equal (50.8 and 49.2%). In p16-positive OPSCC, patients had smaller primary tumors (T1–2), but the N class was more advanced resulting in a higher stage. In addition, p16-positive tumors had a higher histological grade and patients carrying p16-positive tumors were more often nonsmokers. Patients, who had an anterior-wall OPSCC, or a class T4b tumor were most likely to receive definitive oncological treatment. The incidence of p16-positive tumors increased, as during 2000–2004 there were 86 new p16-positive tumors (52.4% of the examined samples) and during 2005–2009 the number was 166 (62.2% of the examined samples). However, the incidence of p16-negative tumors also increased slightly. In addition, treatment changed towards a more oncological approach (Supplementary Figure 1).
Table 1. Baseline clinical characteristics of the 600 oropharyngeal squamous cell carcinoma patients treated with curative intent. p16 staining was available from 431 patients.
Completed treatment with curative intent
Surgery and postoperative oncological treatment
Altogether 270 (47.9%) patients received primary surgery: Open surgery for the primary tumor was performed for 255 patients and endoscopic surgery for 14 patients as the first treatment. One patient underwent surgery to the neck only. The surgical defect was reconstructed with a microvascular tissue transfer or a pedicular flap in 139 (51.5%) and 12 (4.4%) patients. A neck dissection (ND) was performed for 243 (92.4%) patients in pursuance of primary surgery. Twenty-two patients (8.1%) received surgery alone with no adjuvant therapy. Postoperative oncological treatment was given to 248 (91.9%) patients: 142 received RT and 106 CRT. In postoperative RT and CRT, the prescribed median doses for the operated area were 66 and 60 Gy (range, 45–70 Gy and 50–70 Gy). An interruption of postoperative RT occurred in 29 patients. CRT was concomitant in all cases. The chemotherapeutic agent was cisplatin 40 mg/m2 weekly for 85 patients and 18 patients received 100 mg/m2 every third week. One patient received cetuximab and four received other chemotherapeutic agents. Only 57.4% of the patients received all planned cycles of postoperative CT.
Definitive oncological treatment ( ± salvage surgery)
Definitive oncological treatment was given to 294 (52.1%) patients. Two hundred and forty-nine (84.7%) patients received definitive CRT and 45 (15.3%) definitive RT. CRT was concomitant in 246 (98.8%) cases, whereas three patients received RT with adjuvant or neoadjuvant CT. The median prescribed dose to the macroscopic tumor was 70 Gy (range, 60–74 Gy) in definitive CRT and 66 Gy (range, 60–72 Gy) in definitive RT. Ninety patients received conformal 3D RT and 199 IMRT, but in five patients the RT modality remained unknown. RT had to be interrupted in 48 patients. Of patients receiving concomitant CT, 203 received cisplatin 40 mg/m2 weekly, 13 cisplatin 100 mg/m2 every third week, 6 cetuximab without cisplatin, while 21 received another type of CT without cisplatin. Reduction of CT doses or cycles due to side effects or other patient related factors occurred in 95 (38.6%) patients.
Incomplete treatment with curative intent
The treatment was classified incomplete in 36 (6.0%) patients. Of these, 23 patients had advanced OPSCC, Stage III–IV, but no oncological postoperative treatment was offered because of patient-related reasons such as severe comorbidities or patients refusal or previous RT for a former head and neck malignancy. Postoperative RT was initiated, but not completed in one patient. Definitive RT was not carried out entirely up to the prescribed dose in 12 patients.
Palliative treatment
Of all patients, 74 (11.0%) primarily received palliative treatment. The disease stages were as follows: Stage II n = 1; III n = 2; IVA n = 30; IVB n = 17and IVC n = 18. The exact staging was not available for six patients. Palliative RT was delivered to 26 patients, palliative CT to four patients, palliative CRT to three patients and boron neutron capture treatment to one patient. Two patients underwent palliative surgery. A total of 38 patients received symptomatic treatment only. The median survival time in this patient population was 3.2 months (range, 0.1–35.5).
Appearance of residual and recurrent disease
A residual tumor (disease persistence within three months from treatment completion) appeared in 37 patients. Of them, six had undergone primary surgery and 31 had undergone definitive oncological treatment. Out of all the 37 patients, 16 underwent salvage surgery and five of them were alive with no evidence of disease after a median follow-up of five years (range, 4.6–5.0). Twenty-seven patients died of disease and five of other causes. The median survival time among patients with a residual disease was 0.9 years (range, 0.0–4.9).
Altogether 120 patients (20%) developed a recurrent disease within five years and 84% of these occurred within three years. The recurrence was detected first at locoregional site in 76 patients, first at distant site in 38 patients and concurrently at locoregional and distant sites in six patients. A locoregional recurrence developed more often in patients with a p16-negative tumor. A distant recurrence developed nearly as often in patients with a p16- and p16-negative tumor (Supplementary Table 1).
Outcome
Treatment outcome after curative treatment intent
The three- and five-year OS, DSS and DFS rates are shown in . DSS stratified by T class, N class, stage, p16 and treatment are presented in . Patients carrying a p16-positive tumor had a better five-year DSS (81.0%) than those with a p16-negative tumor (57.2%). In addition, the group who underwent primary surgery as part of their treatment had a better five-year DSS (76.3%) compared with to the group receiving definitive oncological treatment (66.7%). We also analyzed the p16- and p16-negative groups separately (): Among the p16-positive subgroup, patients carrying T1–3 tumors had a better DSS than those with T4 tumors. Regarding N class, N1–N2b classes had a minimal impact on survival compared with N0. In the p16-negative subgroup, only patients with a T1 tumor showed a relatively good DSS (three-year DSS 93.0%). DSS was poor regardless of any N class (three-year DSS varying between 76.3–0.0% among the N0–N3 classes). Of patients who underwent primary surgery 39 (18.1%) had positive surgical margins. All these patients received postoperative oncological treatment. We analyzed the effect of surgical margins on DSS. Positive surgical margins did not impair the DSS (Supplementary Figure 2).
Figure 2. Disease specific survival (DSS) curves of oropharyngeal squamous cell carcinoma patients treated with curative treatment intent. (A) DSS of patients according to T class. (B) DSS of patients according to N class. (C) DSS of patients according to stage. (D) DSS of patients according to p16 status and (E) DSS of patients according to treatment approach. Sx + (C)RT: Surgery + (chemo)radiotherapy; CRT ± Sx: Chemoradiotherapy ± Salvage surgery.
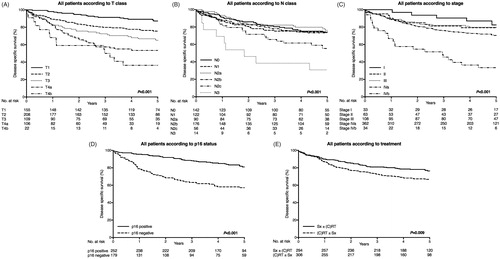
Figure 3. Disease specific survival (DSS) curves of oropharyngeal squamous cell carcinoma patients with p16-positive and negative tumors. Patients with curative treatment intent included (n = 600). (A) DSS of patients with p16-positive disease according to T class. (B) DSS of patients with p16-positive disease according to N class. (C) DSS of patients with p16-positive disease according to stage. (D) DSS of patients with p16-negative disease according to T class. (E) DSS of patients with p16-negative disease according to N class and (F) DSS of patients with p16-negative disease according to stage.
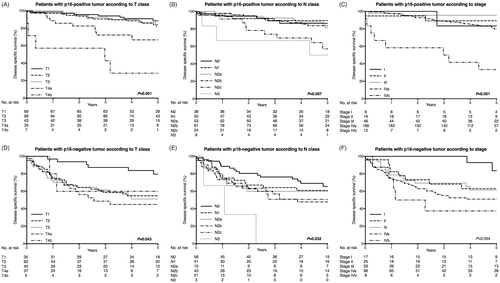
Table 2. The three- and five-year overall (OS), disease-specific (DSS) and disease-free survival (DFS) of patients with curative treatment intent.
Survival in lateral and anterior OPSCC according to treatment approach
Patients with lateral wall tumors treated with Sx + RT and Sx + CRT had a good prognosis; three-year DSS reaching 86.3 and 93.0%. The corresponding figures for definitive CRT or CRT + Sx were 79.0 and 80.4%.() The patients who had anterior wall tumors and received CRT had a better prognosis than those who underwent primary surgery as part of their treatment.
Table 3. The three-year overall (OS), disease specific (DSS) and disease-free survival (DFS) in lateral (tonsillar and tonsillar pillars) and anterior wall (base of tongue and vallecula) oropharyngeal squamous cell carcinoma according to treatment approach.Table Footnotea
Multivariate analysis of various patient, tumor and treatment related factors
Multivariate analysis is presented separately for patients with lateral and anterior wall OPSCC, because a statistically significant interaction occurred between primary tumor localization and treatment (definitive CRT ± salvage Sx vs. Sx + (C)RT) (p = .013), primary tumor localization and RT modality (3D conformal RT vs. IMRT) (p = .001) and primary tumor localization and N class (p = .037). Due to low incidence of superior and posterior wall OPSCC, patients having tumors at these sites were not included in multivariate analysis. In lateral wall OPSCC, p16 negativity, presence of regional metastasis and male sex were associated with increased five-year disease mortality. In anterior wall OPSCC, p16 negativity, 3D conformal RT (in comparison to IMRT), classes T3–4 and male sex were associated with increased five-year disease mortality ().
Table 4. Multivariate cox regression analysis for five-year overall and disease specific survival of patients with lateral (tonsillar and tonsillar pillars) and anterior wall (base of tongue and vallecula) oropharyngeal squamous cell carcinoma.Table Footnotea
Discussion
We conducted a ten-year survey of all OPSCC patients treated at the five university hospitals to evaluate the treatment outcome in a nationwide series of 674 patients. During the study period the annual number of OPSCC patients increased, as a result of increase in the p16-positive cases.
This study is a continuum to the earlier nationwide retrospective cohort study including all patients diagnosed and treated at the five Finnish university hospitals between 1995–1999 [Citation27]. In the earlier series, the five-year DSS of patients with lateral wall tumors was 73%, compared to our present 75%. The five-year DSS of patients with an anterior wall OPSCC had improved slightly from 47 to 65% (). In the 1990s, surgery followed by radiotherapy was the mainstay of treatments for OPSCC patients in Finland, as up to 85% of all patients had surgery for their primary tumors [Citation27]. Consistent with other reports [Citation24,Citation25], during the last years of this study, definitive oncological treatment became a standard (Supplementary Figure 1). Surgery still has a significant role for many of these patients and the actual rate of surgeries did not decrease during the study period. In our material, 49% of patients treated with a curative intent, underwent primary surgery, which despite the changing trends in the management has been suggested to have an important role e.g., in HPV or p16-negative tumors [Citation28,Citation29] and in patients having a history of heavy smoking [Citation29]. During the same period, the number of HPV-related (p16-positive) OPSCC has increased in the Western World [Citation30–32]. This increase may largely explain the improved survival figures, as HPV-related OPSCC has a more favorable prognosis [Citation9]. Introduction of the IMRT technique and CRT in the beginning of the study period may also have impacted the survival figures.
The multivariate analysis of our patient series was performed without patients carrying a T4b tumor or receiving RT or Sx only in order to eliminate patients with obvious selection bias. The analysis revealed three interactions suggesting a separate analysis for lateral- and anterior-wall disease. Both in lateral- and anterior-wall disease p16-positivity seemed to be associated with decreased risk of disease mortality. In addition, patients who had a lateral-wall disease and neck metastasis or were males seemed to have impaired DSS. CRT ± salvage Sx vs. Sx + (C)RT had a HR of 1.8, but the observation remained non-significant (p = .073). However, the finding was significant in a model without the backward elimination. The role of surgery in the management of tonsillar disease may only be speculated and it should be further evaluated in a prospective randomized controlled setting. However, also the possible negative effect of combined vs. single modality treatment needs further investigation. In anterior-wall disease, patients who carried a large tumor (T3–4), were treated with conformal 3D RT (when compared with IMRT) or were males, had a significantly impaired DSS. In anterior-wall disease the main treatment modality (CRT ± salvage Sx vs. Sx + (C)RT) did not have any impact on DSS after adjustment of confounders. Most of these patients received definitive oncological treatment, and only one fourth underwent Sx + (C)RT. The use of Sx + (C)RT in the treatment of anterior wall OPSCC decreased during the study period, as the use of CRT for the same site increased (data not shown). During our study period, robotic surgery was not available and more research is needed to sort out its role, especially in the treatment of small primary tumors. Statistically significant interactions between treatment related factors remained absent.
In our material, positive surgical margins on histology did not have a statistically significant effect on survival (Supplementary Figure 2). All pertinent surgical procedures in the present series were carried out with the aim at achieving clear microscopic margins. Nevertheless, positive surgical margins still occurred in one fifth of the cases. Some earlier reports have also suggested that even non-radical surgery may improve survival in tonsillar SCC [Citation33,Citation34]. We may therefore speculate that oncological treatment may be more efficacious on microscopic residual tumor cells when the primary tumor has been macroscopically resected.
Our results showed that as compared with conventional RT, IMRT was significantly associated with improved outcome among patients with p16-negative tumors (Supplementary Figure 2). Patients with p16-positive tumors had favorable outcome regardless of RT method (Supplementary Figure 2). Likewise, Loimu et al. have previously presented good outcome in patients with a base of tongue OPSCC treated with IMRT [Citation35]. Thus, improved outcome among patients with anterior wall OPSCC might be linked to the IMRT and introduction of CRT and not only to the increase of p16-positive disease. Treatment with definitive CRT also offered a better outcome than only definitive RT, as previously reported comprehensively [Citation36].
Some studies had pointed out that the UICC seventh edition TNM classification of malignant tumors alone reflected poorly OPSCC survival [Citation5,Citation10,Citation21,Citation37]. It had also been suggested, that the TNM staging would establish a prognostic value mainly among HPV-negative patients [Citation38]. However, current eighth edition of the UICC TNM classification of malignant tumors divides OPSCC into two categories according to p16 status, which probably will aid survival estimation in patients belonging either to p16- or to p16-negative subgroups [Citation20]. In the current eighth UICC Edition, among p16-positive OPSCC, classes T4a and b and N1–N2b are combined [Citation20]. Our results indicated that amongst patients with a p16-positive OPSCC, markedly impaired survival was seen among patients with a large primary tumor (T4 class), but although number of patients with a T4b tumor was small, these patients had clearly worse outcome than those with a T4a tumor. The outcome of patients with p16-positive N0–N2b disease was relatively good and the outcome was impaired only among patients with N2c or N3 disease, which matches well with the current eighth edition of UICC TNM classification. However, the number of patients with N3 tumor was also too small to draw any firm conclusions. Among patients with p16-negative OPSCC only those with stage I disease had a relatively good survival rate and the outcome gradually worsened with more advanced T and N classes. Interestingly, those patients who had stage II p16-negative OPSCC clearly had a poor outcome suggesting that they may have been either undertreated or under staged. This same phenomenon, showing poor survival among patients with stage II tumors has previously been observed in studies of laryngeal cancer [Citation39]. Possibly the current N classification for all p16-negative HNSCC aids the survival evaluation in p16-ngeative OPSCC, as the N classification is largely based on the occurrence of extra-nodal extension [Citation20].
Despite national treatment guidelines, the treatment is always individually tailored causing some variations between given treatments. All patients are not suitable for obtaining CRT, and in such cases surgery with postoperative RT may be a more appropriate treatment option. Notably, in about one third of the patients receiving definite CRT it was not possible to administrate all cycles of CT. RT is not considered an optimal treatment for large necrotic lymph nodes, even though RT or CRT might otherwise be the recommended treatment option [Citation40]. The theoretical basis for this is that tumors containing large amounts of hypoxic cells are more resistant to radiotherapy [Citation41]. For those patients presenting with a large necrotic lymph node metastasis-and typically with a small p16-positive primary tumor- surgery, possibly neck dissection (ND) alone, followed by CRT or RT is often carried out at our institutions.
This study presents a large unselected, nationwide and consecutive series of all OPSCC patients treated in Finland. Due to the national health care system, all patients had equal access to treatment and therefore the socioeconomic factors affecting the treatment selection are limited. Furthermore, the five Finnish university hospitals have similar treatment facilities and patient compliance to follow-up is generally good. Due to the retrospective nature of this study, some clinical data, like information on smoking history, remained partly limited. In addition, tumor tissue unavailability limited p16-status determination, as it was available in only 72% of the patients. Our data suffered from these limitations and from lack of a comorbidity or performance index. Therefore, comparison between treatment methods must be done with special caution as selection bias may be present. p16 and smoking status had a significant correlation (Gamma Value =0.808) with each other. In addition, smoking data was fairly limited and especially the number of nonsmokers was limited in anterior-wall disease resulting in extensive confidence intervals. Thus, we excluded smoking status from the multivariate analysis. The p16 status did not have an effect on the chosen treatments since it was very rarely available at the time of treatment decision. In addition, our study remained as survival data evaluation and lacked evaluation of functional outcome.
In conclusion, we demonstrated that from 2000 to 2009, the incidence of OPSCC increased countrywide, which occurred along with the increase in the number of patients with p16-positive tumors. These patients had a better survival rate than those with p16-negative tumors, which is in accordance with previous studies. Along with the increased incidence, treatment had changed towards a more oncological approach. In anterior-wall disease a decrease in the rate of surgical treatment with concurrent improvement in the outcome was observed. This improved outcome was not only mainly associated with p16 positivity but was also associated with developments in oncological treatment. In lateral-wall disease, the rate of surgical treatment remained higher throughout the study period. The role of surgery in the management of OPSCC disease requires further investigation.
Lauri_et_al._Supplementary_material.zip
Download Zip (315.1 KB)Acknowledgments
We express our gratitude to the colleagues at the Finnish Central Hospitals who provided essential follow-up data on several patients. Special thanks to: Satu Tommola, Katriina Kostamo, Heikki Teppo, Panu Rajala and Hannu Tapiovaara. We thank Mr. Tero Wahlberg for the statistical assistance.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619.
- Hakulinen T, Tryggvadottir L, Gislum M, et al. Trends in the survival of patients diagnosed with cancers of the lip, oral cavity, and pharynx in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49:561–577.
- Braakhuis BJ, Visser O, Leemans CR. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: increasing incidence, but not in young adults. Oral Oncol. 2009;45:e85–e89.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559.
- Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128–142.
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720.
- Allen CT, Lewis JS, Jr., El-Mofty SK, et al. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope. 2010;120:1756–1772.
- Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472.
- Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820.
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
- Hong AM, Dobbins TA, Lee CS, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer. 2010;103:1510–1517.
- Lassen P, Eriksen JG, Krogdahl A, et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol. 2011;100:49–55.
- Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967–2980.
- Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269.
- Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006; 24:5630–5636.
- Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope 2012; 122(Suppl 2):S13–S33.
- Mehanna H, Evans M, Beasley M, et al. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S90–SS6.
- Pfister DG, Spencer S, Adelstein D, et al. Head and Neck Cancers Version 2.2017. Natl Compr Canc Netw. 2017;15:761–770.
- Samuels SE, Eisbruch A, Beitler JJ, et al. Management of locally advanced HPV-related oropharyngeal squamous cell carcinoma: where are we? Eur Arch Otorhinolaryngol. 2016; 273:2877–2894.
- Brierley JD,K, GM, Wittekind C, editors. TNM Classification of Malignant Tumours. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2016.
- Rietbergen MM, Brakenhoff RH, Bloemena E, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann Oncol. 2013; 24:2740–2745.
- Holsinger FC, Ferris RL. Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: robotics, lasers, and clinical trials. J Clin Oncol. 2015;33:3285–3292.
- Mydlarz WK, Chan JY, Richmon JD. The role of surgery for HPV-associated head and neck cancer. Oral Oncol. 2015;51:305–313.
- Chen AY, Schrag N, Hao Y, et al. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117:16–21.
- Chen AY, Zhu J, Fedewa S. Temporal trends in oropharyngeal cancer treatment and survival, 1998–2009. Laryngoscope. 2014;124:131–138 .
- Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th ed. Hoboken, NJ: Wiley-Blackwell; 2011.
- Makitie AA, Pukkila M, Laranne J, et al. Oropharyngeal carcinoma and its treatment in Finland between 1995–1999: a nationwide study. Eur Arch Otorhinolaryngol. 2006;263:139–143.
- Wang MB, Liu IY, Gornbein JA, et al. HPV-positive oropharyngeal carcinoma: a systematic review of treatment and prognosis. Otolaryngol Head Neck Surg. 2015;153:758–769.
- Seikaly H, Biron VL, Zhang H, et al. Role of primary surgery in the treatment of advanced oropharyngeal cancer. Head Neck. 2016;38(Suppl 1):E571–E579.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301.
- Rietbergen MM, Leemans CR, Bloemena E, et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. 2013;132:1565–1571.
- Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 2009;125:362–366.
- Holliday MA, Tavaluc R, Zhuang T, et al. Oncologic benefit of tonsillectomy in stage I and II tonsil cancer. JAMA Otolaryngol Head Neck Surg. 2013;139:362–3666.
- Yildirim G, Morrison WH, Rosenthal DI, et al. Outcomes of patients with tonsillar carcinoma treated with post-tonsillectomy radiation therapy. Head Neck. 2010;32:473–480.
- Loimu V, Collan J, Vaalavirta L, et al. Patterns of relapse following definitive treatment of head and neck squamous cell cancer by intensity modulated radiotherapy and weekly cisplatin. Radiother Oncol. 2011;98:34–37.
- Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14.
- Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119:81–89.
- Ward MJ, Mellows T, Harris S, et al. Staging and treatment of oropharyngeal cancer in the human papillomavirus era. Head Neck. 2015;37:1002–1013.
- Haapaniemi A, Koivunen P, Saarilahti K, et al. Laryngeal cancer in Finland: a 5-year follow-up study of 366 patients. Head Neck. 2016;38:36–43.
- Paximadis PA, Christensen ME, Dyson G, et al. Up-front neck dissection followed by concurrent chemoradiation in patients with regionally advanced head and neck cancer. Head Neck. 2012;34:1798–1803.
- Begg AC. Predicting recurrence after radiotherapy in head and neck cancer. Semin Radiat Oncol. 2012;22:108–118.