Ramucirumab is an intravenously administered human monoclonal antibody (IgG1) that selectively binds the extracellular domain of vascular endothelial growth factor receptor 2 (VEGFR2), blocking the pro-angiogenic effects of vascular endothelial growth factor (VEGF) [Citation1–3]. It has been approved by the US Food and Drug Administration (FDA) to treat advanced or metastatic gastric or gastroesophageal junction adenocarcinoma with disease progression during or after pretreatment with platinum-containing or fluoropyrimidine-containing chemotherapy, metastatic colorectal cancer in combination with irinotecan plus 5-fluorouracil and metastatic non-small cell lung cancer in combination with docetaxel [Citation1,Citation2].
Cutaneous toxicities caused by VEGF and VEGFR inhibitors include hand–foot skin reaction, mucositis, pruritus, skin exanthema, facial edema, subungual splinter hemorrhages, alopecia, hair or skin discoloration, eruptive melanocytic nevi and increase the incidence of keratoacanthoma and squamous cell carcinoma [Citation3]. Otherwise, vascular tumors are not a known skin complications of these drugs [Citation2].
Cherry angiomas (CA), also known as senile angiomas or Campbell de Morgan spots, are the most common benign vascular proliferation [Citation4–7]. The term eruptive cherry angiomas (ECA), which involves the sudden and extensive onset of multiple CA, has been rarely reported and its etiopathogenesis has been poorly investigated [Citation4–7]. We report a patient who developed ECA while receiving ramucirumab.
Case report
In July 2014, a 66-year-old man was diagnosed with stage IV HER2-negative gastric adenocarcinoma, with peritoneal and liver metastases. After first-line chemotherapy with fluorouracil and cisplatin and owing to the disease progression, second-line chemotherapy was started: ramucirumab (8 mg/kg) was administered intravenously every 2 weeks and paclitaxel (80 mg/m2) was administered intravenously once a week during 3 weeks of every 28-day cycle.
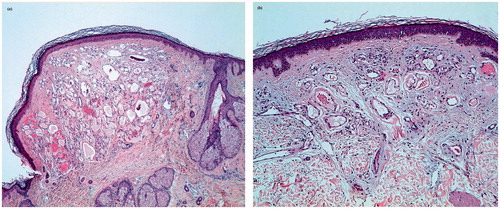
After 3 out of these cycles, he developed between 40 and 50, 2–5 mm, smooth, dome-shaped, bright-red-vascular-appearing papules on his chest (), abdomen (), back () and scalp (). An eroded similar papule was seen on the tip of his tongue (). The patient reported that the lesions were new and sudden in onset. He had no fever. Punch biopsies of 2 lesions from the trunk and the scalp were performed. They showed dilated vascular channels filled with erythrocytes, located on the upper-mid-dermis, lined by endothelial cells (). Serological tests for hepatitis B, C, human immunodeficiency virus, syphilis, cytomegalovirus, Epstein-Barr virus, parvovirus, Borrelia burgdorferi and rickettsia were negative. Computerized axial tomography demonstrated no angiomas in the liver or in the spine. On the basis of the clinical and histopathological findings, a diagnosis of ECAs was made. No therapy was recommended.
Figure 1. Multiple cherry angiomas on the chest and abdomen (a), back (b) and scalp (c). An eroded cherry angioma on the tongue (d).
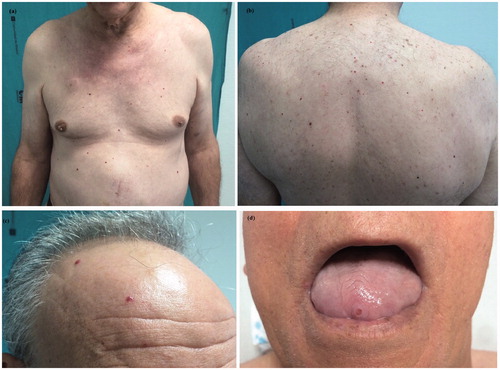
Figure 2. Histopathology of a cherry angioma on the scalp (a) and on the trunk (b) showing dilated vascular channels fully filled with erythrocytes and scant intervening stroma. The epidermis is thinned and surrounds the angioma as a collarette in (a) (hematoxylin-eosin stain, original magnification ×10 (a), ×10 (b)).
In August 2015, after 4 cycles of paclitaxel-ramucirumab, the patient was hospitalized due to an acute upper gastrointestinal bleeding which required 3 blood units. Consequently, ramucirumab was discontinued and paclitaxel treatment was kept. Four months later, the patient had been admitted to a palliative care center due to a general deterioration in his overall health and a progression of his disease so he did not attend his appointment in our center. Otherwise, a paliativist in the palliative center was contacted and he reported that cherry angiomas had improved, as the number of lesions had been reduced, no new lesions had been developed and the angioma on the tongue had been resolved after the withholding of ramucirumab
Discussion
ECA have been linked to topical nitrogen mustard [Citation4,Citation5,Citation8], bromides [Citation7], cyclosporine [Citation6], chronic graft host disease [Citation9] and lymphoproliferative diseases [Citation10]. Borghi et al. found advanced age, cutaneous and extra-cutaneous tumors and immunosuppressive treatments as highly significant risk factors for the ECA [Citation4]. They suggest that the imbalance of skin immune competence could be a predisposing factor for the development of ECA [Citation4].
Recently Lim et al. described a patient who had developed a spontaneous proliferative angioma with a somatic activating VEGFR2 mutation in the setting of ramucirumab therapy [Citation2]. Clinical examination showed a single 40 mm, well-demarcated blanchable red plaque on his shin [Citation2]. Histological features resembling a tufted angioma [Citation2]. The authors suggest that vascular lesions may represent paradoxical VEGFR activation in the setting of ramucirumab therapy [Citation2].
Based on our observation, we propose that ECA in our patient should be induced by ramucirumab. The sudden appearance of vascular lesions upon treatment with ramucirumab and the improvement of them after the withholding of ramucirumab supports our consideration. We also consider immunosuppression caused by chemotherapy and cancer disease might have played a role in our patient’s course. Although further studies are warranted, medical oncologist and dermatologists should be aware of this possible complication induced by ramucirumab treatment.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Vennepureddy A, Singh P, Rastoqi R, et al. Evolution of ramucirumab in the treatment of cancer - A review of literature. J Oncol Pharm Pract. 2017;23:525–539.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240–1243.
- Ishak RS, Aad SA, Kyei A, et al. Cutaneous manifestations of anti-angiogenic therapy in oncology: Review with focus on VEGF inhibitors. Crit Rev Oncol Hematol. 2014;90:152–164.
- Borghi A, Minghetti S, Battaglia Y, et al. Predisposing factors for eruptive cherry angiomas: new insights from an observational study. Int J Dermatol. 2016;55:e598–e600.
- MA HJ, Zhao G, Shi F, et al. Eruptive cherry angiomas associated with vitiligo: provoked by topical nitrogen mustard? J Dermatol. 2006;33:877–879.
- De Felipe I, Redondo P. Eruptive angiomas after treatment with cyclosporine in a patient with psoriasis. Arch Dermatol. 1998;134:1487–1488.
- Cohen AD, Cagnano E, Vardy DA. Cherry angiomas associated with exposure to bromides. Dermatology (Basel). 2001;202:52–53.
- Zhu LL, Zheng S, Wei H, et al. Multiple cutaneous malignancies and cherry hemangiomas in a vitiligo patient treated with topical nitrogen mustard. Dermatol Ther. 2014;27:52–54.
- Soo JK, Mortimer PS. Eruptive angiomas associated with graft-versus-host disease. Br J Dermatol. 2006;154:376–378.
- Fajgenbaum DC, Rosenbach M, van Rhee F, et al. Eruptive cherry hemangiomatosis associated with multicentric Castleman disease. Jama Dermatol. 2013;149:204–208.
