Abstract
Purpose: The standard of care for locally advanced bladder cancer (LABC) is neoadjuvant chemotherapy followed by cystectomy. However, the role of adjuvant therapy is unclear. The purpose of this study was to evaluate the outcomes of adjuvant chemotherapy for patients with LABC following neoadjuvant chemotherapy and cystectomy, and to determine whether select patients may benefit from adjuvant chemotherapy.
Methods: The National Cancer Data Base (NCDB) was queried (2004–2013) for patients with newly diagnosed pT3-4N0-3M0 bladder cancer that received neoadjuvant chemotherapy and cystectomy. Patients were divided into two groups based on the adjuvant therapy they received: chemotherapy alone or observation. Statistics included multivariable logistic regression to determine factors predictive of receiving adjuvant chemotherapy, Kaplan–Meier analysis to evaluate overall survival (OS) and Cox proportional hazards modeling to determine variables associated with OS.
Results: Altogether, 2592 patients met inclusion criteria; 901 (34.8%) patients received adjuvant chemotherapy, while 1691 (65.2%) were observed. Patients treated with adjuvant chemotherapy were more likely to have positive margins were younger and more likely to receive treatment at a nonacademic facility. There was no difference in median OS between patients treated with or without adjuvant chemotherapy (22.6 vs. 21.1 months; p = .267). However, a longer median OS was observed with the use of adjuvant chemotherapy was observed among patients with N2–3 disease (17.5 vs. 14.4 months; p = .005) and positive surgical margins (16.7 vs. 12.2 months; p = .025). On multivariate analysis, advancing age, pT4 stage, positive N stage, positive margins and lower socioeconomic status were associated with worse OS.
Conclusions: In the largest study to date evaluating efficacy of adjuvant chemotherapy, while no difference in OS was observed for adjuvant chemotherapy in all patients, a longer OS was observed among patients with N2–3 disease or with positive surgical margins. Prospective studies are recommended to further evaluate these findings.
Introduction
Approximately 25% of bladder cancer patients present with muscle invasive disease [Citation1]. For these patients, radical cystectomy has long been a part of the typical treatment regimen. However, given the high rate of distant failure for these patients, systemic chemotherapy was explored as a treatment option. Randomized data found that neoadjuvant chemotherapy added to cystectomy resulted an improvement in overall survival (OS) for these patients [Citation2–4]. In addition, a meta-analysis found an OS benefit of approximately 5% with the addition of neoadjuvant chemotherapy [Citation5]. As a result of level 1 evidence, neoadjuvant chemotherapy followed by radical cystectomy is the standard of care recommendation per the National Comprehensive Cancer Network (NCCN) [Citation6].
However, the use for adjuvant chemotherapy is less clear. For patients who did not receive neoadjuvant chemotherapy prior to radical cystectomy, adjuvant chemotherapy is often used in the United States as an alternative [Citation7]. Randomized trials of adjuvant chemotherapy after cystectomy were often not well-designed and did not find meaningful benefits [Citation8]. A meta-analysis of trials did find a reduction in the risk of death for patients receiving adjuvant chemotherapy following cystectomy [Citation8]. However, this analysis included a very inhomogeneous group of patients and was limited to patients who had not received neoadjuvant chemotherapy prior to undergoing radical cystectomy.
As a result, the role of adjuvant chemotherapy in patients having undergone neoadjuvant chemotherapy is even more uncertain. In addition to a survival benefit, neoadjuvant systemic therapy results in clinical downstaging. However, despite this, 20% of patients still have adverse pathologic features at the time of cystectomy [Citation9]. Adjuvant chemotherapy for those with residual adverse features after neoadjuvant chemotherapy has not been a significant topic of discussion. To our knowledge, no study has examined the practice patterns and effect of adjuvant chemotherapy for all patients with locally advanced bladder cancer (LABC). To determine the use, role and benefit of adjuvant chemotherapy in patients who received neoadjuvant chemotherapy prior to cystectomy, we evaluated the large, contemporary National Cancer Data Base (NCDB).
Material and methods
This investigation analyzed the NCDB, which is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de-identified information regarding tumor characteristics, patient demographics and patient survival for approximately 70% of the US population [Citation10–12]. The NCDB contains information not included in the surveillance, epidemiology and end results database, including details regarding use of systemic therapy and radiation dose. The data used in the study were derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
The most recently released NCDB dataset corresponded to the years 2004–2013. Inclusion criteria for this study involved patients age ≥18 with bladder cancer treated with neoadjuvant chemotherapy and cystectomy and found to have pT4/3pN0-3M0 disease. For inclusion, patients required histological diagnostic confirmation, definitive treatment with neoadjuvant chemotherapy and cystectomy (surgical procedure of the primary site codes 50, 60–64, 70–74, 80) and a recorded vital status. Patients treated with radiation therapy were excluded from this analysis, because this is not standard therapy in the neoadjuvant chemotherapy/cystectomy paradigm [Citation6]. Patients were divided into two cohorts: those receiving adjuvant chemotherapy and those observed following surgery. Using a classification scheme from other published studies utilizing the NCDB, an academic facility was defined as an institution with both an accession of more than 500 newly diagnosed cancer cases per year and one that provided postgraduate medical education in at least four program areas, including internal medicine and general surgery [Citation13]. All other facilities, including Comprehensive Community Cancer Programs, Community Cancer Programs and Integrated Network Programs were categorized as nonacademic, as none of these institutions requires graduate medical education.
Information collected on each patient included demographic data, comorbidity information, clinicopathologic tumor parameters and treatment facility characteristics. All statistical tests were two-sided, with a threshold of p < .05 for statistical significance and were performed using STATA version 14 (StataCorp, College Station, TX, USA). Fisher’s exact or χ2 test analyzed categorical proportions between groups in the non-parametric and parametric settings, respectively. Univariable and multivariable logistic regression modeling were utilized to determine characteristics that were predictive for receipt of adjuvant chemotherapy. The Kaplan–Meier method was used for survival analysis and comparisons between the two treatment paradigms were performed with the log-rank test for all patients. Subset analysis was performed while stratifying patients by nodal status and surgical margins status. OS was defined as the interval between the date of diagnosis and the date of death or last contact. Multivariate Cox proportional hazards modeling was additionally used to identify variables associated with OS in the entire cohort. Patients included in the multivariate analysis were those found to be statistically significant on univariate analysis.
Results
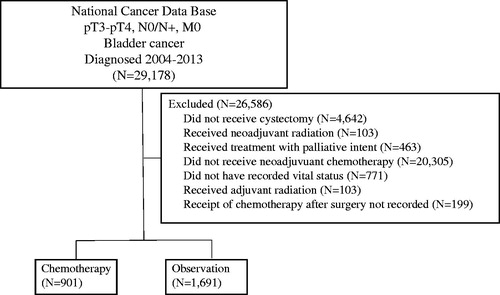
A complete flow diagram of patient selection is provided in . In total, 2592 patients met inclusion criteria. Of these, 901 (34.8%) patients received adjuvant chemotherapy, while 1691 (65.2%), the majority of patients, were observed. The median follow up for all patients was 25.5 months (interquartile range, 15.4–42.8 months). displays clinical characteristics of the analyzed patients. The majority of patients were Caucasian, male, < 65 years of age and with urothelial histology. Multivariable logistic regression analysis was performed to evaluate factors independently associated with receiving adjuvant chemotherapy. Patients treated with adjuvant chemotherapy were more likely to have positive margins, were younger and more likely to receive treatment at a nonacademic facility. These factors are summarized in .
Table 1. Demographic and clinical characteristics.
Table 2. Multivariable logistic regression for factors predictive of use of adjuvant chemotherapy.
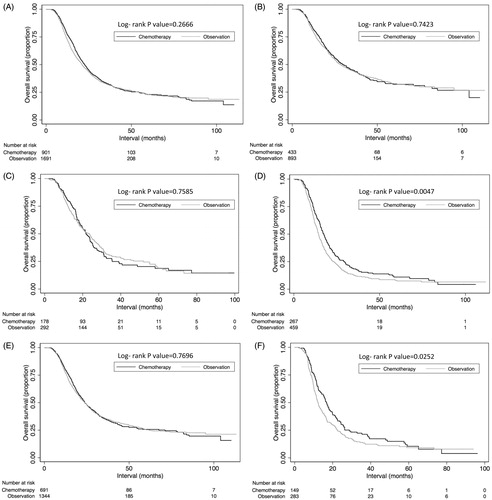
On univariate analysis using log-rank testing, there was no difference in median OS between patients treated with or without adjuvant chemotherapy (22.6 vs. 21.1 months; p = .2666). However, on subset analysis, a longer median OS was observed with the use of adjuvant chemotherapy among patients with N2–3 disease (17.5 vs. 14.4 months; p = .0047) and positive surgical margins (16.7 vs. 12.2 months; p = .0252). Survival outcomes are presented in .
Figure 2. Kaplan–Meier overall survival curves comparing those receiving adjuvant chemotherapy versus observation for all patients (A); among node negative patients (B); among pN1 patients (C); among N2–3 patients (D); among patients with negative surgical margins (E); and among patients with positive surgical margins (F).
On multivariate Cox proportional hazards modeling analysis, advancing age, pT4 stage, positive N stage, positive margins and lower socioeconomic status were associated with worse OS. Higher socioeconomic status was associated with better OS. These prognostic factors are presented in . Multivariate analysis was not performed in the N2–N3 or margin positive subsets due to the relatively small sample sizes of each of these cohorts.
Table 3. Univariate and multivariate analysis for factors predictive of overall survival.
Discussion
Given the lack of clear evidence for adjuvant chemotherapy for bladder cancer, evaluating a large contemporary database like the NCDB is important to evaluate national practice patterns and potential avenues to continually improve outcomes. To our knowledge, this is the largest study to date examining the use, role and benefit of adjuvant chemotherapy after neoadjuvant chemotherapy and radical cystectomy. Several observations can be made from this analysis. First, in accordance with the existing literature, observation is the most common management strategy after neoadjuvant chemotherapy and cystectomy, even with residual adverse pathologic findings. However, unsurprisingly, adjuvant chemotherapy was more likely to be in the setting of positive margins, where patients are at a higher risk of recurrence. Another intuitive finding was that adjuvant chemotherapy was more likely to be given in younger patients, who would be more likely to tolerate additional systemic therapy. Finally, while it was not surprising that advancing age, higher T stage, higher N stage, positive margins, it must be noted that lower economic status was also on multi-variate analysis associated with worse outcomes on multivariate analysis.
This is the largest study to date to describe the practice patterns associated with adjuvant chemotherapy use for patients with LABC receiving both neoadjuvant chemotherapy and radical cystectomy. On multivariate logistic regression, receipt of treatment at a nonacademic facility was associated with a decreased likelihood of adjuvant chemotherapy use. This is most likely due to the lack of clear evidence describing the indications for adjuvant chemotherapy use for patients with LABC receiving neoadjuvant chemotherapy. In fact, the NCCN guidelines only recommend adjuvant chemotherapy use in instances where neoadjuvant chemotherapy has not been used [Citation6]. Additionally, there was a nonsignificant trend for patients N1 or N2 disease to be predictive for chemotherapy use, likely due to the perceived increased risk for distant failure or metastasis with node positive disease. The fact that no such association was observed for patients with N3 disease may have been due to the relatively small number of patients in the study with N3 disease.
In terms of survival outcomes, there was no difference in OS between the observation group and the adjuvant chemotherapy group as a whole, in line with findings from a single institution retrospective review and two randomized studies showing no OS improvement with adjuvant chemotherapy when used adjuvantly for patients with LABC [Citation14–16]. However, on subset analysis, patients with positive margins or N2+ disease were observed to have a longer OS with the use of adjuvant chemotherapy. This finding may suggest that, as has been demonstrated in T4b bladder cancer and in large non-small cell lung cancer, there may be certain high risk populations that may derive more benefit from the use of adjuvant chemotherapy [Citation17,Citation18]. Therefore, it is possible that certain subsets of high-risk patients with LABC, such as those with N2–3 disease or positive margins, due to their high risk of recurrence, may benefit more from the use of adjuvant chemotherapy. Of course, prospective randomized data is needed to definitively determine the role of adjuvant chemotherapy in high-risk patients. Unfortunately, the relative rarity of LABC makes trial enrollment difficult and necessitates the analysis of large databases for clues on potentially new treatment strategies.
Our study has several limitations due to its reliance on the NCDB. First, the retrospective nature of the study and all associated inherent biases must be acknowledged. This study does not preclude the need for the gold standard of randomized clinical trials. Second, the NCDB does not keep track of several noteworthy variables, such as reasons for a particular treatment, receipt of targeted therapies and salvage treatments. In addition, the NCDB does not record other endpoints such as tolerance of therapy, cancer-specific survival and local/regional control, which are important variables when assessing the utility of additional systemic therapy. Moreover, patients treated with radiation therapy were excluded from this study, suggesting that we do not know the role of adjuvant chemotherapy in patients who have either previously used radiation therapy or the role of adjuvant chemotherapy when used as a radiosensitize with radiation therapy. While this study was not designed to determine the efficacy of radiation therapy, there may be potential benefits in use of radiation therapy for select high risk patients, particularly in the setting of positive surgical margins and future studies should further explore this question [Citation19,Citation20]. However, these limitations do not diminish the need for further investigations of the role of adjuvant chemotherapy in the treatment of LABC.
Conclusions
In the treatment of LABC, there was no OS benefit with adjuvant chemotherapy after radical cystectomy and neoadjuvant chemotherapy in all patients. However, for patients with N2+ disease or positive margins, a longer media OS was observed in the subset of patients receiving adjuvant chemotherapy. On multivariate analysis, advancing age, pT4 stage, positive N stage, positive margins and lower socioeconomic status were associated with worse OS. This study is the largest study of adjuvant chemotherapy to date and our findings highlight the need for additional prospective study to determine the patient population most likely to benefit from adjuvant treatment, with special attention given to high risk groups such as patients with N2–3 disease or positive surgical margins.
Disclosure statement
There are no acknowledgements. This study has not been presented, published in part or full form elsewhere. All authors declare no conflicts of interest.
Additional information
Funding
References
- Howlander N, Noone AM, Krapcho M, et al. Seer cancer statistics review, 1975–2009 (vintage 2009 populations). Bethesda (MD): National Cancer Institute; 2012.
- Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354:533–540.
- Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the ba06 30894 trial. J Clin Oncol. 2011;29:2171–2177.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866.
- Collaboration ABCM-a. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927–1934.
- National Comprehensive Cancer Network [Internet]. Bladder Cancer. Version 5.2017; 2017. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
- Feifer A, Taylor JM, Shouery M, et al. Multi-institutional quality-of-care initiative for nonmetastatic, muscle-invasive, transitional cell carcinoma of the bladder: phase I. J Clin Oncol. 2011;29:240–240.
- Raghavan D, Bawtinhimer A, Mahoney J, et al. Adjuvant chemotherapy for bladder cancer-why does level 1 evidence not support it? Ann Oncol. 2014;25:1930–1934.
- Yafi FA, Aprikian AG, Chin JL, et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: a canadian multicentre experience. BJU Int. 2011;108:539–545.
- Bilimoria KY, Stewart AK, Winchester DP, et al. The national cancer data base: a powerful initiative to improve cancer care in the united states. Ann Surg Oncol. 2008;15:683–690.
- Haque W, Verma V, Butler EB, et al. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol. 2017;133:369–375.
- Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: analysis of the national cancer data base. Gynecol Oncol. 2017;144:28–33.
- Brower JV, Chen S, Bassetti MF, et al. Radiation dose escalation in esophageal cancer revisited: a contemporary analysis of the national cancer data base, 2004 to 2012. Int J Radiat Oncol Biol Phys. 2016;96:985–993.
- Zargar-Shoshtari K, Kongnyuy M, Sharma P, et al. Clinical role of additional adjuvant chemotherapy in patients with locally advanced urothelial carcinoma following neoadjuvant chemotherapy and cystectomy. World J Urol. 2016;34:1567–1573.
- Freiha F, Reese J, Torti FM. A randomized trial of radical cystectomy plus cisplatin, vinblastine, and methotrexate chemotherapy for muscle invasive bladder cancer. J Urol. 1996;155:495–500.
- Cognetti F, Ruggeri FM, Felici A, et al. Adjvuant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian multicenter, randomized phase III trial. Ann Oncol. 2012;23:695–700.
- Haque W, Verma V, Butler EB, et al. National practice patterns and outcomes for T4b urothelial cancer of the bladder. Clin Genitourin Cancer. 2017;S1558–7673:30268–30269.
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:146–154.
- Christodouleas JP, Hwang WT, Baumann BC. Adjuvant radiation for locally advanced bladder cancer? A question worth askin. Int J Radiat Oncol Biol Phys. 2016;94:1040–1042.
- Reddy AV, Pariser JJ, Pearce SM, et al. Patterns of failure after radical cystectomy for pT3-4 bladder cancer: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:703–706.