Abstract
Background: Whole brain radiotherapy (WBRT) is considered standard of care for patients with multiple brain metastases or unfit for radical treatment modalities. Recent studies raised discussion about the expected survival after WBRT. Therefore, we analysed survival after WBRT for brain metastases ‘in daily practice’ in a large nationwide multicentre retrospective cohort.
Methods: Between 2000 and 2014, 6325 patients had WBRT (20 Gy in 4 Gy fractions) for brain metastases from non-small cell lung cancer (NSCLC; 4363 patients) or breast cancer (BC; 1962 patients); patients were treated in 15 out of 21 Dutch radiotherapy centres. Survival was calculated by the Kaplan–Meier method from the first day of WBRT until death as recorded in local hospital data registration or the Dutch Municipal Personal Records Database.
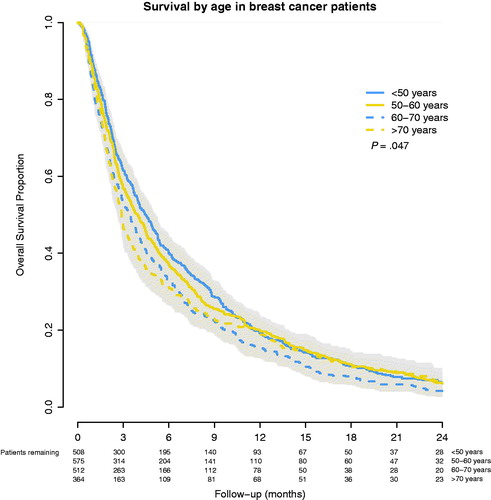
Findings: The median survival was 2.7 months for NSCLC and 3.7 months for BC patients (p < .001). For NSCLC patients aged <50, 50–60, 60–70 and >70 years, survival was 4.0, 3.0, 2.8 and 2.1 months, respectively (p < .001). For BC patients, survival was 4.5, 3.8, 3.2 and 2.9 months, respectively (p = .047). In multivariable analyses, higher age was related to poorer survival with hazard ratios (HR) for patients aged 50–60, 60–70 and >70 years being 1.05, 1.19 and 1.34, respectively. Primary BC (HR: 0.83) and female sex (HR: 0.85) were related to better survival (p < .001).
Interpretation: The survival of patients after WBRT for brain metastases from NSCLC treated in Dutch ‘common radiotherapy practice’ is poor, in breast cancer and younger patients it is disappointingly little better. These results are in line with the results presented in the QUARTZ trial and we advocate a much more restrictive use of WBRT. In patients with a more favourable prognosis the optimal treatment strategy remains to be determined. Prospective randomized trials and individualized prognostic models are needed to identify these patients and to tailor treatment.
Introduction
Brain metastases occur in 10–30% of patients with metastasized solid tumours [Citation1]. In non-small lung cancer (NSCLC) and breast cancer, this incidence is even higher [Citation2,Citation3]. With higher incidences of cancer and improving local tumour control due to improved local and systemic therapies, the incidence of brain metastases will likely increase. For patients with a limited number of brain metastases and with a reasonable performance status, radical treatment with either surgery or stereotactic radiosurgery (SRS) is currently considered the standard of care [Citation4]. For all other patients, whole brain radiotherapy (WBRT) is considered the standard of care.
Throughout the years, many radiotherapy dose escalation studies have been performed, with disappointing results [Citation5]. None showed an overall survival (OS) benefit, despite more toxicity and a worse quality of life (QoL) after higher doses of radiotherapy [Citation6,Citation7]. Therefore, 20 Gy in five fractions is considered the most patient friendly treatment schedule.
The use of WBRT has been justified by one small randomized study wherein WBRT (20 × 2 Gy) was compared with best supportive care (BSC) [Citation8]. This study included 48 patients with brain metastases from various primary tumours. The median survival was slightly better in the WBRT group (3.3 versus 2.3 months), but no formal statistical analysis was performed and QoL was not investigated. Given the small sample size, no conclusions can be drawn about the effectiveness of WBRT.
Therefore, the British/Australian QUARTZ trial was ground-breaking, as it randomised a large group of patients (538) with brain metastases from NSCLC that were treated with WBRT along with BSC, and BSC alone. Patients were eligible if they were unfit for more radical treatment – for example, SRS or surgery – and if the effectiveness of WBRT was questioned in this specific patient. This non-inferiority study showed no evidence for a difference between the two study arms in QoL, dexamethasone use or survival (median 9.2 weeks after BSC plus WBRT, versus 8.2 weeks after BSC alone) [Citation9].
These relatively unexpected results were criticized because OS was supposedly poorer than the alleged survival in daily radiotherapy practice, suggesting selection bias [Citation10,Citation11]. Indeed, only 6% of included patients had a favourable RPA class I and about a third of the included patients had a Karnofsky performance status <70.
At the end of the last century, three recursive partitioning analysis (RPA) classes in patients with brain metastases were identified [Citation12]. The RPA class is a predictor for OS in patients with brain metastases; with a median survival of 7 months in RPA class I, dropping to 2 months in RPA class III [Citation5]. The more recent diagnosis specified-graded prognostic assessment (DS-GPA) outperformed the initial GPA, but also found a much lower OS of only 3.6 months [Citation13].
A second point of criticism on the QUARTZ trial results was that patients with a more favourable primary tumour, such as breast cancer, could maybe benefit more from WBRT. Several studies which included patients with brain metastases from various primary tumours, describe higher median survivals after WBRT, namely between 2 and 6 months [Citation14–16]. However, trial populations may not always be a representative sample of the total population due to restrictive trial eligibility criteria and may therefore provide a biased picture of ‘daily radiotherapy practice’ [Citation17].
In the present study, we tried to support the aforementioned two criticisms raised against the QUARTZ study. We investigated survival in patients with brain metastases from NSCLC and breast cancer treated with WBRT in ‘daily radiotherapy practice’ using a large multicentre retrospective cohort in the Netherlands.
Methods
Out of the 21 Dutch radiotherapy institutions, 15 academic and non-academic centres with a cancer registration participated in this retrospective survival analysis of all patients with brain metastases from NSCLC or breast cancer (BC) who received WBRT between January 2000 and December 2014.
Data were retrieved from the hospitals’ local data registration systems because no Dutch nationwide registration for patients with brain metastases exists. The following data were recorded: age at time of treatment, year of treatment, sex, primary tumour site, date of treatment and date of death/last follow-up. No thorough information was available on metastatic load (i.e., number and volumes of brain metastases). If the last follow-up date was not available, the date of death was locally retrieved from the Dutch Municipal Personal Records Database. All data were retrieved in 2016 or 2017 to ensure a minimum follow up of at least 12 months. The anonymized patient data were collected in a central database.
Radiotherapy
All patients received 20 Gy external beam radiotherapy in five fractions of 4 Gy. Two (conventional or IMRT) opposed lateral fields were applied with a linear accelerator using 6 MeV or 10 MeV photons. The target volume included at least the entire brain. Radiation fields could be extended to include the lower border of the body of vertebra C2. Treatment position was supine and a thermoplastic fixation mask was used to minimize movement.
Statistical analyses
All data were analysed using the statistical package IBM SPSS Statistics for Windows 20.0 (IBM Corporation, Armonk, NY, USA). Continuous and categorical variables were summarized by descriptive statistics. Descriptive data are given as a mean (±standard deviation) or median (range). Patients were grouped into four age-groups: <50, 50–60, 60–70 and ≥70 years. The treatment period was divided into three periods: 2000–2006, 2007–2010 and 2011–2014. Periods were chosen in accordance with the wide availability of trastuzumab (2007) and EGFR mutation analyses (2011) in the Netherlands. Furthermore, in 2011 a new guideline was implemented urging a more restrictive use of WBRT in patients with poor performance. Overall survival was calculated from the start of radiotherapy until the date of death or last follow-up alive, using the Kaplan–Meier method with the log rank test to determine significance. Data were censored at the last follow-up for patients still alive. Univariate and multivariable Cox proportional hazards models were fit to evaluate the impact of factors predictive of survival. The following factors were used in multivariable analyses: treating institute, primary tumour, age group and treatment period. All testing was two-tailed with .05 as level of significance.
Results
Patients
A total of 6325 patients treated with WBRT for brain metastases of NSCLC or breast cancer with retrievable data were identified. Of these, 4363 had brain metastases from NSCLC (69%) and 1962 from primary breast cancer (31%). The median age was 62 years (range 23–92 years) for the entire cohort, 58 years (range 23–92 years) for the breast cancer group and 63 years (range 26–90 years) for the NSCLC group. The patient characteristics are reported in . One institution was only able to recollect data on breast cancer patients and two institutions were unable to identify sex from their datasets.
Table 1. Patient characteristics and median overall survival of NSCLC and breast cancer patients with brain metastases who were treated with WBRT.
Overall survival
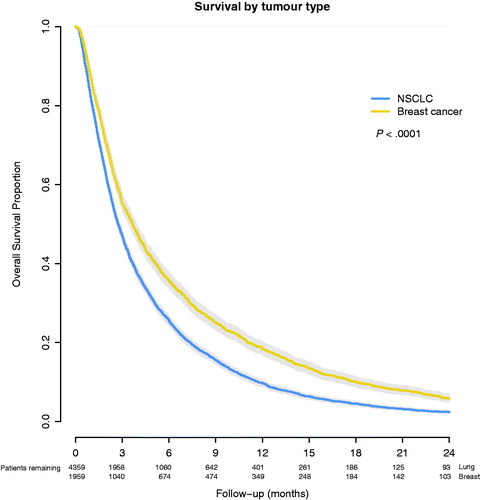
The median survival was 2.7 months (95% CI: 2.6–2.8 months) for NSCLC patients and 3.7 months (95% CI: 3.4–3.9 months) for breast cancer patients (p < .001) (). Survival at 2, 6, 12 and 24 months was 62%, 26%, 10% and 2%, respectively, for the patients with NSCLC. For breast cancer patients, survival at 2, 6, 12 and 24 months was 70%, 36%, 19% and 6%, respectively. In univariate analyses, survival was correlated to primary tumour, age, sex, treatment period and treating institute (, and ).
Figure 2. Overall survival after WBRT for brain metastases from primary NSCLC, stratified by age-group.
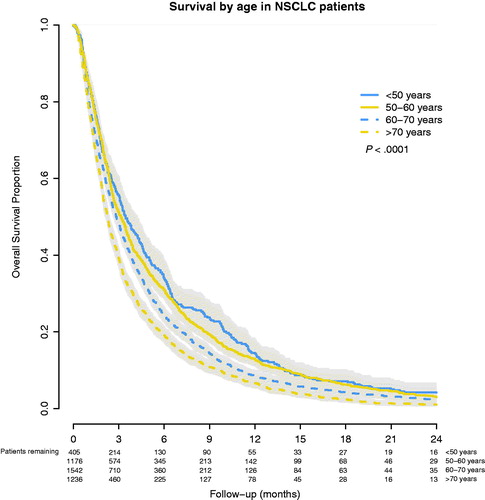
Figure 3. Overall survival after WBRT for brain metastases from primary BC, stratified by age group.
Multivariable analyses results are reported in . NSCLC and old age had a statistically significant worse survival while female sex had a statistically significant better survival.
Table 2. Multivariable analyses for overall survival of NSCLC and breast cancer patients with brain metastases who were treated with WBRT.
Discussion
Survival after WBRT in this large cohort of Dutch patients with brain metastases in ‘standard radiotherapy practice’ is poor and comparable to that found in the QUARTZ-study, and is considerably worse than in most other prospective studies. The survival of patients with brain metastases from breast cancer is somewhat better than from NSCLC. Elderly patients do worse than younger patients, both in breast cancer and in NSCLC. Similarly, male patients do worse than female patients.
Apart from the QUARTZ trial, survival in prospective clinical trials on brain metastases treated with WBRT in NSCLC tend to be higher than we reported with a median survival of 5.2–7.2 months and even higher when combined with systemic therapy [Citation3]. However, in cohort studies overall survival tends to be 2–3 months, which is very comparable to our findings [Citation15,Citation18].
Survival for patients with brain metastases from breast cancer is only marginally better compared to NSCLC (2.7 versus 3.7 months). Our assumption that patients with brain metastases of a tumour type with a more favourable prognosis would profit more from WBRT is an argument not supported by evidence.
A matched pair comparison between WBRT and BSC for brain metastasis did not find a correlation between tumour type and benefit from WBRT [Citation16]. Only patients with brain metastasis from SCLC, a known radiosensitive disease, had a significantly better survival after WBRT than after BSC [Citation16].
We found a statistically significant correlation between age and survival, as was found in the QUARTZ trial. The median age in our group (62 years) was similar to that in the QUARTZ trial (66 years). The impact of age on survival seems larger in NSCLC compared to breast cancer patients, which has been observed previously [Citation19,Citation20].
We found a significantly better survival in women with NSCLC (3.2 months, N = 1622) compared with men (2.5 months, N = 2137). This difference has been attributed to differences in epidemiology, biology, genetics, sex-related hormonal factors and treatment responses [Citation21]. For breast cancer, male numbers were too low for conclusions. In the QUARTZ data no significant difference for sex was seen. As no multivariable analyses were reported and patients were not stratified by age, no conclusions can be drawn from that study concerning the effect of sex on the outcome after WBRT.
Over the years, systemic treatment has slightly increased the survival of patients with metastatic disease. However, we did not see an improvement in OS over time in our cohorts. Perhaps this is due to a limited effect of systemic treatment on brain metastases because of poor passage of the blood brain barrier. Another explanation could be that with the increase of SRS, patients currently treated with WBRT are in fact more likely to be at the end stage of the disease than in the past, and only few will receive systemic treatment. As we did not have data on the time between diagnose of brain metastases and the start of WBRT, we could not evaluate such a potential effect.
According to the Dutch guidelines from 2011, patients were eligible for SRS or surgery if their RPA class I was or II, they had a maximum of three brain metastases of maximum 3.5–4 cm each and if they had limited or treatable extracranial disease. As this was a consensus guideline, it likely reflected daily practice in the Netherlands for the previous years. The aforementioned guideline explicitly advises refraining from WBRT in patients with an RPA class III. In daily practice, still 11–21% of patients receiving WBRT are in RPA class III and only 6% are in RPA class I [Citation20,Citation22]. In the QUARTZ study also 6% were in RPA class I. Because of the low prevalence of RPA class I in both series, we cannot make any recommendation on this group. Although a more aggressive approach is often advocated, little if any evidence exists for its efficacy.
There is a trend towards more use of SRS. In most clinical guidelines, SRS is indicated in up to three brain metastases, although the few RCTs in SRS for brain metastases did not show a survival advantage in patients with more than one metastasis [Citation7,Citation23]. Nevertheless, up to 10 metastases are reported to have been successfully treated with SRS [Citation24]. Unfortunately, these studies are predominantly single centre studies without a control group. Although best local control is seen when combining WBRT and SRS, this had a detrimental effect on QoL and neurocognitive functioning [Citation25]. A recently opened European study randomizes between SRS and WBRT in patients with four or more brain metastases [Citation26]. With a primary endpoint of QoL, it will be interesting to see whether SBRT can maintain QoL in this patient group.
In the QUARTZ trial, WBRT did not improve the QoL or change the use of dexamethasone [Citation9]. Although an indirect measure, dexamethasone use could be considered a surrogate for clinically relevant symptoms of brain metastases. QoL is known to deteriorate over time after WBRT [Citation27–29]. This may in part be due to disease progression, however WBRT itself may have a detrimental effect on QoL, due to both acute toxicity and late toxicity [Citation7,Citation30]. Given the generally poor survival in this patient group, acute toxicity seems most relevant. WBRT has a negative effect on both fatigue and QoL in the first two months after treatment [Citation29]. To date there is no study showing the benefit of WBRT on symptoms such as headaches or neurological deficits. As it does not seem to affect survival either, the question arises if there is any benefit from WBRT for the majority of patients. In our cohort we see a small subgroup of patients that have a substantial longer survival.
Strengths and limitations
Since we had no data on intracranial tumour burden, extracranial disease status or performance status, we were unable to compute RPA classes or (DS)GPA-scores. We also did not have information on EGFR or ALK mutation status in NSCLC patients, which reportedly has a substantial impact on survival in patients with brain metastases from NSCLC [Citation3]. Likewise, there was no subdivision possible in the various types of breast cancer. Furthermore, due to the absence of QoL data, a temporary beneficial effect by WBRT cannot be excluded. Finally, as we analysed only the patients treated with 5 × 4 Gy, a hypothetical selection bias could have occurred where patients with a presumed better prognosis might have received a higher dose with a more fractionation schedule, despite the lack of evidence available at that time [Citation31]. In the Netherlands, more protracted schedules have only exceptionally been given, and have never been included in the national guidelines. Nevertheless, this could have impacted our findings.
However, our study includes a large number of patients with data from the last 15 years of 75% of Dutch radiotherapy institutions. Hence, we believe that our data reliably represents the outcomes of WBRT in daily clinical practice.
Conclusion
The survival of patients after WBRT for brain metastases from NSCLC treated in Dutch ‘common radiotherapy practice’ is poor, especially in the elderly. Survival for breast cancer patients, whose disease is expected to result in a more prolonged prognosis, is disappointingly little better. These results are in line with the results presented in the QUARTZ trial and we advocate a much more restrictive use of WBRT. In patients with a more favourable prognosis the optimal treatment strategy remains to be determined. Prospective randomized trials and individualized prognostic models are needed to identify these patients and to tailor treatment.
Ethics committee approval
The study has been approved by the Leids University Medical Centre Medical Ethics committee, Leiden, The Netherlands.
Disclosure statement
We declare no conflict of interests.
Additional information
Funding
References
- Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7:337–344.
- Law A, Karp DD, Dipetrillo T, et al. Emergence of increased cerebral metastasis after high-dose preoperative radiotherapy with chemotherapy in patients with locally advanced nonsmall cell lung carcinoma. Cancer. 2001;92:160–164.
- Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev. 2016;45:139–162.
- IKNL-Integraal Kankercentrum Nederland/Comprehensive Cancer Centre the Netherlands. Oncoline Cancer Clinical Guidelines: Hersenmetastasen (Guideline Brain Metastasis). 2011.
- Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. In: Tsao MN, editor. Cochrane database of systematic reviews. Chichester (UK): John Wiley & Sons, Ltd; 2012.
- Khanduri S, Gerrard G, Barton R, et al. Clinical trials assessing the optimal management of brain metastases – the state of play. Clin Oncol. 2006;18:744–746.
- Scoccianti S, Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol. 2012; 102:168–179.
- Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971;111:334–336.
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority. Lancet. 2016;388:2004–2014.
- Pechoux CL, Dhermain F, Besse B. Whole brain radiotherapy in patients with NSCLC and brain metastases. Lancet. 2016;388: 1960–1962.
- Bruynzeel AME, Lagerwaard FJ. Whole brain radiotherapy for brain metastases from non-small cell lung cancer: the end of an era? J Thorac Dis. 2016;8:E1525–E1527.
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751.
- Nieder C, Andratschke NH, Geinitz H, et al. Diagnosis-specific graded prognostic assessment score is valid in patients with brain metastases treated in routine clinical practice in two European countries. Med Sci Monit. 2012;18:CR450–CR455.
- Tsao MN, Sultanem K, Chiu D, et al. Supportive care management of brain metastases: what is known and what we need to know. Conference proceedings of the National Cancer Institute of Canada (NCIC) Workshop on Symptom Control in Radiation Oncology. Clin Oncol. 2003;15:429–434.
- Wong E, Tsao M, Zhang L, et al. Survival of patients with multiple brain metastases treated with whole-brain radiotherapy. CNS Oncol. 2015;4:213–224.
- Nieder C, Norum J, Dalhaug A, et al. Radiotherapy versus best supportive care in patients with brain metastases and adverse prognostic factors. Clin Exp Metastasis. 2013;30:723–729.
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence – what is it and what can it tell us? N Engl J Med. 2016;375: 2293–2297.
- Windsor AA, Koh E-S, Allen S, et al. Poor outcomes after whole brain radiotherapy in patients with brain metastases: results from an international multicentre cohort study. Clin Oncol. 2013; 25:674–680.
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425.
- Lutterbach J, Bartelt S, Stancu E, et al. Patients with brain metastases: hope for recursive partitioning analysis (RPA) class 3. Radiother Oncol. 2002;63:339–345.
- Paggi MG, Vona R, Abbruzzese C, et al. Gender-related disparities in non-small cell lung cancer. Cancer Lett. 2010;298:1–8.
- Hendriks LEL, Troost EGC, Steward A, et al. Patient selection for whole brain radiotherapy (WBRT) in a large lung cancer cohort: impact of a new Dutch guideline on brain metastases. Acta Oncol. 2014;53:945–951.
- Khan M, Lin J, Liao G, et al. Comparison of WBRT alone, SRS alone, and their combination in the treatment of one or more brain metastases: review and meta-analysis. Tumour Biol. 2017;39:1010428317702903.
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395.
- Soliman H, Das S, Larson DA, et al. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7:12318–12330.
- Zindler JD, Bruynzeel AME, Eekers DBP, et al. Whole brain radiotherapy versus stereotactic radiosurgery for 4-10 brain metastases: a phase III randomised multicentre trial. BMC Cancer. 2017;17:500.
- Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer. 2002;38:487–496.
- Chow E, Davis L, Holden L, et al. Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage. 2005;30:18–23.
- Pulenzas N, Khan L, Tsao M, et al. Fatigue scores in patients with brain metastases receiving whole brain radiotherapy. Support Care Cancer. 2014;22:1757–1763.
- Soffietti R, Kocher M, Abacioglu UM, et al. A European organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31:65–72.
- Tsao MN, Lloyd N, Wong RK, et al. 2006. Whole brain radiotherapy for the treatment of multiple brain metastases. In: Tsao MN, editor. Cochrane database of systematic reviews. Chichester (UK): John Wiley & Sons, Ltd.