Abstract
Aim: To our knowledge, no prior studies have addressed the possible effects of tumour height on the accuracy of preoperative magnetic resonance imaging (MRI)-based staging relative to postoperative histopathological assessments in patients with adenocarcinoma of the rectum (RC). This study aimed to investigate whether the accuracy of preoperative MRI stage in RC is influenced by tumour height.
Methods: A total of 489 consecutive RC patients scheduled for curative treatment between 2009 and 2013 were included. Of the 489 patients, 133 patients had preoperative chemoradiotherapy (CRT), and 356 patients underwent primary surgery. Low, mid and high RC were defined as a tumour <5 cm, 5–10 cm and >10 cm from the anal verge, respectively. Diagnostic MRI and, for patients with CRT, re-staging MRI features including tumour T-stage (mrT), distance between the tumour border and the distance to the mesorectal fascia (mrMRF), extramural tumour depth (mrEMD), extramural vascular invasion (mrEMVI) and nodal involvement (mrN) were correlated with the corresponding postoperative histopathological findings.
Results: There were 115, 186 and 188 patients with low RC, mid RC and high RC, respectively. For all patients, the correlations between mrT and pT and between mrMRF and pCRM were not influenced by tumour height. None of the correlations between mrEMD, mrEMVI and mrN and the corresponding postoperative histopathological findings significantly differed for tumours of different heights. For patients with CRT, a remarkable proportion with low RC were overstaged as ymrT3 compared to ypT0-2.
Conclusions: The ability to preoperatively use MRI to accurately stage is not influenced by tumour height. For patients with preoperative CRT, low RC may be MRI overstaged due to post-radiation fibrosis. We found that mrEMD predicts pEMD reliably and should therefore be considered in treatment decisions. Although new MRI techniques are emerging, preoperative RC staging remains incompletely definitive in daily clinical practice.
Introduction
In clinical settings, the rectum is divided into upper (10–15 cm), mid (5–10 cm) and lower (0–5 cm) sections based on distances measured from the anal verge via rigid proctoscopy and magnetic resonance imaging (MRI). Diagnostic tumour staging is performed using MRI of the lower abdomen and pelvis and CT of the thorax and abdomen. Guiding treatment strategies, accurate descriptions of tumour height, TNM stage (where T, N and M represent tumour T-stage, nodal involvement and metastatic spread, respectively) and, particularly in low rectal cancer, tumour invasion into adjacent organs and the anal sphincter have to be clarified prior to surgery [Citation1].
Patients with adenocarcinoma of the rectum (RC) in the upper part of the rectum are often treated with surgery alone, whereas patients with RC in the middle part of the rectum are often treated with preoperative chemoradiotherapy (CRT) or short-course radiotherapy (SCRT) and surgery. Finally, tumours in the lower part of the rectum are commonly treated with preoperative CRT or SCRT and more extensive surgery (abdominoperineal excision (APE) or extralevator abdominoperineal excision (ELAPE)). These treatment schedules reflect the need for different surgical procedures to achieve a radical resection margin [Citation2]. It is still questioned whether preoperative MRI variables can predict the gold standard of postoperative verification of histopathological variables.
Despite treatments tailored to tumour height and preoperative TNM stage, for RC, the local recurrence rate remains approximately 5–6%, and overall survival (OS) after five years is approximately 60% [Citation3–5]. In particular, patients with low RC have the highest local recurrence rate of up to 20% and the lowest 5-year OS of 47% [Citation6].
Thus, it appears worthwhile to investigate whether the accuracy of preoperative MRI staging for RC is influenced by tumour height.
Material and methods
Patients
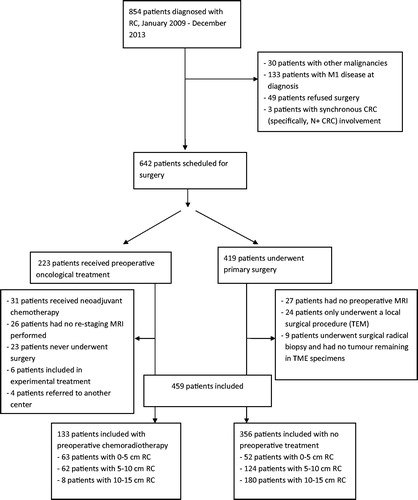
All patients diagnosed with RC in the North Denmark Region between 2009 and 2013 (n = 854), based on data acquired from the national cancer registry, were screened for inclusion. Information concerning treatment was obtained from patient files. In total, 489 patients were found to be suitable for inclusion (). Out of these 489 patients, 133 underwent CRT.
Diagnostic staging
The diagnostic workup comprised of a complete colonoscopy, a biopsy of the suspected tumour, rigid proctoscopy to measure tumour height from the anal verge; MRI of the lower abdomen and pelvis, as well as a CT scan of the thorax/abdomen to assess the TNM stage.
Based on diagnostic staging, the patients were treated as follows (). In total, 419 patients were suitable for primary surgery, and 223 patients were considered for CRT, according to the following guidelines: all T3/T4 tumours in low RC, all T3/T4 tumours in mid RC with mrMRF less than 5 mm and, finally, non-resectable T4 tumours in high RC [Citation7] were treated with preoperative CRT. The distribution of patients with and without CRT according to the tumour height is shown in .
Table 1a. Correlations between mrT and pT and between mrCRM and pCRM for RC of different heights for patients without CRT.
Table 1b. Correlations between ymrT and ypT for RC of different heights in patients with CRT.
Radiological and histopathological investigations
Preoperative MRI was performed on 1.5 T MRI scanners (for detailed scan protocol, see Supplementary Material), and the images were evaluated by a total of four expert GI radiologists. The following MRI-based metrics were recorded according to international clinical guidelines: depth of tumour infiltration within and outside the rectal wall, T-stage (mrT), distance between the tumour border and the distance to the mesorectal fascia (mrMRF), extramural tumour depth (mrEMD), extramural vascular invasion (mrEMVI) and N stage (mrN) [Citation8]. The mrEMD findings were used to divide patients into two groups: T3 < 5 mm and T3 > 5 mm [Citation9,Citation10].
Patients treated with preoperative CRT were re-staged by means of MRI according to national guidelines with mrT, mrEMVI and mrN. The re-staging MRI was conducted 6 weeks after CRT. YmrEMD and ymrMRF were not reported as standard assessments during the study period.
All patients underwent surgery performed in accordance with national guidelines [Citation11].
Histopathological assessments, both macro- and microscopically, were made by two expert GI pathologists according to Leeds guidelines [Citation12]. The TNM staging was diagnosed according to the WHO version 5.0 [Citation13].
All metrics evaluated via preoperative MRI (mrT, mrMRF, mrEMD, mrEMVI and mrN) and re-staging MRI (ymrT, ymrEMVI and ymrN) were correlated with the corresponding postoperative histopathological findings ((y)pT, pCRM, pEMD, (y)pEMVI and (y)pN, respectively). All data were collected in a study database.
The study was approved by the applicable national data protection agency (2008-58-0028).
Statistics
Correlations between MRI-based metrics and the corresponding postoperative histopathological findings, such as mrT versus pT and mrMRF versus pCRM, were evaluated using weighted kappa statistics [Citation14,Citation15]. To compare weighted kappa statistics from the present study with those of other studies, we applied the same weighting matrix to all relevant studies and presented the results as forest plots (see Supplementary Material). Furthermore, correlations between mrEMD and pEMD, between mrEMVI and pEMVI and between mrN and pN were analysed using kappa statistics [Citation16]. No influence on a metric by tumour height was concluded if the 95% confidence interval for the weighted Kappa statistics overlapped. All analyses were conducted with the statistical software Stata, version 11 (StataCorp, College Station, TX, USA).
Results
Comparisons between preoperative mrT and pT and between mrMRF and pCRM according to tumour height are summarised in for patients without CRT. shows the comparison between preoperative ymrT and ypT for patients with CRT. Among the ypT0/T1/T2, there were 8/15 in mid RC and 10/37 in low RC with ypT0. The correlations between ymrT and ypT were not influenced by tumour height. However, there was a tendency towards overstaging in low RC for patients with CRT.
Patients were diagnosed during five years, from 2009 to 2013. The ability to predict the histopathological T stage using preoperative MRI did not exhibit improvement over time (see Supplementary Material).
The correlations between MRI-based measurements (mrEMD, mrEMVI and mrN) and the corresponding histopathological findings for tumours of different heights are shown in for patients without preoperative CRT. For patients with preoperative CRT, the correlations between the re-staging MRI-based measurements (ymrV and ymrN) and the corresponding histopathological findings for tumours of different heights are shown in . These correlations did not markedly differ for tumours of different heights for any of these metrics.
Table 2a. Correlations between MR metrics (mrEMD, mrEMVI and mrN) and the corresponding histopathological findings for RC patients without CRT of different tumour height.
Table 2b. Correlations between MRI metrics (ymrEMVI and ymrN) and the corresponding histopathological findings for RC patients with CRT of different tumour height.
Discussion
To our knowledge, no prior studies have addressed the accuracy of preoperative MRI-based staging relative to postoperative histopathological assessments for RC with respect to tumour height.
For patients without preoperative CRT
In five published studies, irrespective of RC tumour height, broad discordance between mrT and pT was found (calculated weighted kappa correlation values of 0.17–0.83, 95% CI –0.17 to 1.19 (see section “Statistics”)) [Citation17–22]. In the present study, the correlation between mrT and pT was weak (weighted kappa of 0.4, 95% CI 0.31–0.49) and was not influenced by tumour height. The poor correlation between mrT and pT is largely due to pT3 tumours being staged as mrT2, a phenomenon that predominantly occurs in low RC. A possible explanation for this discrepancy could be the well-known difficulties associated with using MRI to describe minor extramural tumour invasion [Citation19]. As recently published, an MRI approach involving designating resection planes as safe or unsafe has been suggested for low RC [Citation23]. Compared with MRI, endoscopic ultrasound (EUS) may provide additional information [Citation24,Citation25]; consequently, at our centre, all low RCs are now staged using both MRI and EUS.
The poor correlation between mrMRF and pCRM found for all types of RC was not correlated with tumour height. These results are consistent with the findings of an earlier published study [Citation26]. However, several earlier studies showed excellent agreement between mrMRF and pCRM [Citation22]. An explanation for the extremely weak correlation between mrMRF and pCRM could be that the MRI measurements are obtained in vivo, whereas the postoperative histopathological findings are generated by examining the surgical removed, formalin-fixed, specimen. It is well known that formalin-fixation contributes to shrinkage of the specimens. For low RC in particular, a likely explanation for the weakness of this correlation is that mrMRF is measured from the invasive tumour border to the mesorectal or intersphincteric fascia, whereas pCRM depends on which type of operation has been performed (intersphincteric, conventional or extralevator APE).
A strong correlation between mrEMD and pEMD was observed for all tumours, irrespective of tumour height; this finding is consistent with the results reported in the literature [Citation10,Citation19,Citation26]. It has been claimed that mrEMD is the most reliable and reproducible metric in preoperative MRI staging [Citation27]. The MERCURY study showed that mrEMD <5 mm is an excellent prognostic factor for local recurrence and disease-free survival (DFS) [Citation27]. As suggested by others [Citation28], patients with mrT3 tumours with mrEMD >5 mm should be recognised as being at high risk of local recurrence and reduced DFS and OS. This group might benefit from preoperative treatment.
Vascular invasion (mainly venous invasion) found by a pathologist is a main clinical prognostic factor in the spread of haematogenous tumours. In our study, we found considerable disagreement between mrEMVI and pEMVI, irrespective of tumour height. In a large proportion of cases for which mrEMVI was positive, pEMVI was negative. The discrepancy between mrEMVI and pEMVI is a multifactorial phenomenon, and further investigation is needed [Citation29–31].
In agreement with previous studies [Citation32], our investigation found a poor correlation between mrN and pN, irrespective of tumour height. Nevertheless, mrN continues to be listed as an important factor in treatment planning and in ongoing trial protocols [Citation33,Citation34].
For patients with preoperative CRT
The correlation between ymrT and ypT according to tumour height showed a remarkable proportion of patients overstaged as ymrT3 and ymrT4 but reported as ypT0-2 for low RC. This finding could be explained by the appearance of post-radiation fibrosis interpreted as parenchymal tumours at the re-staging MRI [Citation35].
A strength of this study is that it involved a homogeneous, consecutive and relatively large cohort of RC patients treated at a single centre. These patients were diagnosed and treated using standardised procedures. Another strength of this study is that all assessments were performed by four specialised MRI radiologists and two specialised pathologists.
A limitation of this investigation is the low number of patients in the subgroups of high RC in preoperative CRT and low RC in patients without preoperative CRT; thus, no conclusions could be drawn for such patients.
Conclusions
The ability to accurately stage RC preoperatively via MRI using mrT, mrMRF, mrEMVI, mrEMD and mrN is not influenced by tumour height for patients without preoperative CRT. For patients with preoperative CRT, low RC may be overstaged at the re-staging MRI due to post-radiation fibrosis. We found that mrEMD appears to predict pEMD reliably and should therefore be considered in treatment decisions to avoid over- or undertreatment. Although new MRI techniques are emerging, preoperative RC staging remains incompletely definitive in daily clinical practice.
Laurids_Ostergaard_Poulsen_et_al._Supplementary_files.zip
Download Zip (427.5 KB)Acknowledgments
During the project period, Dr. Ljungmann was working at the Department of Surgery, Aalborg University Hospital, Aalborg, Denmark.
Disclosure statement
The authors report no conflicts of interest.
References
- Benson AB, Bekaii-Saab T, Chan E, et al. Rectal cancer. J Natl Compr Canc Netw. 2012;10:1528–1564.
- Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi81–vi88.
- Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696–701.
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989.
- Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–346.
- Chiang J, Hsieh P, Chen J, et al. Rectal cancer level significantly affects rates and patterns of distant metastases among rectal cancer patients post curative-intent surgery without neoadjuvant therapy. World J Surg Oncol. 2014;12:197.
- Neoadjuverende behandling af resektabel rectumcancer. [Internet]; Available from: http://dccg.dk/retningslinjer/20140418/2014_NeoAdjRectum.pdf (in Danish).
- Taylor FGM, Swift RI, Blomqvist L, et al. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827–1835.
- Merkel S, Mansmann U, Siassi M, et al. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298–304.
- MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132–139.
- DCCG.dk. National Danish Surgical Guidelines 2010–2014. [Internet]; Available from: http://dccg.dk/wp-content/uploads/2017/08/2010_rectumkirgenerelt.pdf
- Virtual Pathology at the University of Leeds. [Internet]; Available from: http://www.virtualpathology.leeds.ac.uk/
- Sobin LH, Wittekind CH. TNM classification on malignant tumours. 5th ed. New York: Wiley-Liss Publications; 1997.
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363.
- Maclure M, Willett WC. Misinterpretation and misuse of the kappa statistic. Am J Epidemiol. 1987;126:161–169.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276–282.
- Oberholzer K, Junginger T, Kreitner KF, et al. Local staging of rectal carcinoma and assessment of the circumferential resection margin with high-resolution MRI using an integrated parallel acquisition technique. J Magn Reson Imaging. 2005;22:101–108.
- Akasu T, Linuma G, Takawa M, et al. Accuracy of high-resolution magnetic resonance imaging in preoperative staging of rectal cancer. Ann Surg Oncol. 2009;16:2787–2794.
- Brown G, Radcliffe AG, Newcombe RG, et al. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364.
- Halefoglu AM, Yildirim S, Avlanmis O, et al. Endorectal ultrasonography versus phased-array magnetic resonance imaging for preoperative staging of rectal cancer. WJG. 2008;14:3504–3510.
- Branagan G, Chave H, Fuller C, et al. Can magnetic resonance imaging predict circumferential margins and TNM stage in rectal cancer? Dis Colon Rectum. 2004;47:1317–1322.
- Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–2223.
- Battersby NJ, How P, Moran B, et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II study. Ann Surg. 2016;263:751–760.
- Cârţână ET, Gheonea DI, Săftoiu A. Advances in endoscopic ultrasound imaging of colorectal diseases. World J Gastroenterol. 2016;22:1756–1766.
- Tudyka V, Blomqvist L, Beets-Tan RGH, et al. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014;40:469–475.
- Pedersen BG, Moran B, Brown G, et al. Reproducibility of depth of extramural tumor spread and distance to circumferential resection margin at rectal MRI: enhancement of clinical guidelines for neoadjuvant therapy. AJR Am J Roentgenol. 2011;197:1360–1366.
- Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711–719.
- Hunter CJ, Garant A, Vuong T, et al. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol. 2012;19:1199–1205.
- Chand M, Evans J, Swift RI, et al. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. 2015;261:473–479.
- Chand M, Palmer T, Blomqvist L, et al. Evidence for radiological and histopathological prognostic importance of detecting extramural venous invasion in rectal cancer: recommendations for radiology and histopathology reporting. Colorectal Dis. 2015;17:468–473.
- Chand M, Rasheed S, Heald R, et al. Adjuvant chemotherapy may improve disease-free survival in patients with mrEMVI-positive rectal cancer following chemoradiation. Colorectal Dis. 2017;19:537–543.
- Iafrate F, Laghi A, Paolantonio P, et al. Preoperative staging of rectal cancer with MR imaging: correlation with surgical and histopathologic findings. Radiographics. 2006;26:701–714.
- Glynne-Jones R, Hava N, Goh V, et al. Bevacizumab and Combination Chemotherapy in rectal cancer Until Surgery (BACCHUS): a phase II, multicentre, open-label, randomised study of neoadjuvant chemotherapy alone in patients with high-risk cancer of the rectum. BMC Cancer. 2015;15:764.
- Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer – the RAPIDO trial. BMC Cancer. 2013;13:279.
- Blazic IM, Campbell NM, Gollub MJ. MRI for evaluation of treatment response in rectal cancer. Br J Radiol. 2016;89:20150964.