Abstract
Background: The long-term toxicities after cisplatin-based chemotherapy (CBCT) reveal a remarkable inter-individual variation among testicular cancer survivors (TCSs). Therefore, we assessed long-term platinum (Pt) changes and their associations with CBCT-related late effects in TCSs.
Material and methods: In 77 TCSs treated with CBCT from 1984 to 1990, blood samples for analyses of Pt and a questionnaire including self-reported neuro- and ototoxicity (NTX) symptoms were collected during two follow-up surveys at median 12 (Survey I; SI) and 20 (Survey II; SII) years after treatment. Information about second cancers after SII was retrieved from the Norwegian Cancer Registry.
Results: A larger Pt decline from SI to SII was associated with a decreased risk of a second cancer diagnosis (HR 0.78, 95% CI 0.62–0.99 per 10 ng/L/year), and worsening of paresthesias in hands (OR 1.98, 95% CI 1.09–3.59 per 10 ng/L/year) and tinnitus (OR 1.51, 95% CI 1.01–2.27 per 10 ng/L/year).
Conclusion: In summary, we found a significant association between a larger Pt decline and a reduced risk of second cancers and deterioration of paresthesias in hands and tinnitus.
Background
The 5-year cancer-specific survival rate of Norwegian patients with testicular cancer (TC) currently exceeds 97% [Citation1], and even the majority of men with advanced disease are cured after cisplatin-based chemotherapy (CBCT). TC survivors (TCSs) are at risk of several well-described long-term (developing during/shortly after treatment) and late effects (becoming apparent months to years after treatment has ended) [Citation2] associated with CBCT. These include second cancers, cardiovascular disease (CVD) and neuro- and ototoxicity (NTX) symptoms [Citation3,Citation4].
Clinicians have observed large inter-individual variations in treatment-related toxicities among patients treated with comparable chemotherapy (CT) regimens. However, apart from treatment burden and some genetic polymorphisms [Citation5], it is presently difficult to identify patients at particularly high risk for long-term effects after CBCT.
Cisplatin can be retained in the human body for decades [Citation6–9]. Higher cumulative cisplatin doses are positively associated with higher serum platinum (Pt) levels measured up to 28 years after treatment [Citation7]. Approximately 50% of cisplatin will be eliminated from the body within the first five days after administration. Further on, the elimination of cisplatin can presumably be described by numerous half-lives, which increase with time [Citation10,Citation11]. Between 120 and 240 months after cisplatin administration the half-live is estimated to be 54 months [Citation12].
Furthermore, ex vivo experiments have shown that up to 10% of circulating Pt remains reactive [Citation10], but the underlying mechanisms regarding Pt retention, and why some individuals retain more Pt than others remains unresolved [Citation13]. How Pt is retained and how its changes over time contribute to ongoing tissue damage has not been elucidated. Most of the published studies in this field have had a cross-sectional design with few exceptions [Citation8,Citation14], and longitudinal studies are necessary to reveal associations between CBCT, long-term Pt change and treatment-related side effects.
Therefore, the aims of the present longitudinal study were to (1) quantify long-term changes in serum Pt levels by assessments at median 12 and 20 years after treatment, and (2) explore the associations between the long-term Pt change and the risk of second cancers, renal function and NTX in TCSs treated with CBCT.
Material and methods
Study population, design and treatment
A national multicenter follow-up survey, performed at five university hospitals, invited all Norwegian long-term survivors of unilateral germ cell TC aged 18 to 75 years and treated in the period 1980–1994. Of 1814 eligible men, 1463 (81%) participated in Survey I (SI) (1998–2002) [Citation15]. Exclusion criteria included bilateral orchiectomy for any reason, extragonadal germ cell cancer, other malignancies except skin cancer and mental retardation. A second survey (SII) (2007–2008) was conducted among 1093 of the same TCSs (80% of eligible men, ). SI and SII included a physical examination and venipuncture for blood sampling at the hospital or general practitioner (GP), as well as a comprehensive questionnaire.
Figure 1. Overview of survivors of testicular cancer included in Survey I (1998–2002), Survey II (2007-2008) and present study.
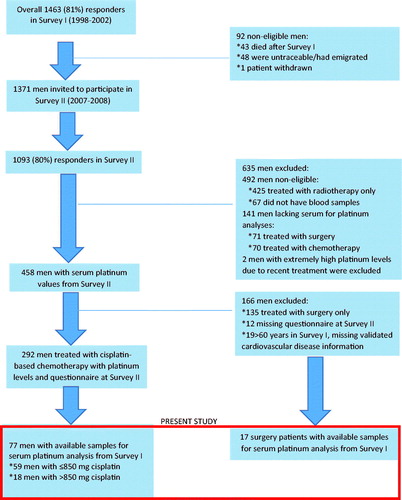
The present longitudinal study comprises 77 cases and 17 controls treated with CBCT and surgery only, respectively. These 94 TCSs represent all participants with available Pt measurements at both SI and SII (). The 17 controls were included to evaluate any long-term change in Pt levels in TCSs not exposed to CBCT. The Committee for Medical Research Ethics, Southern Health Region of Norway, approved both Surveys and all patients had given written informed consent.
After orchiectomy and staging according to the Royal Marsden Hospital System [Citation16], cisplatin was combined with bleomycin and either etoposide (BEP) or vinblastine (CVB) in the majority of patients with metastatic disease. Principles for the cytotoxic treatment of TC in Norway between 1980 and 1994 have been described previously [Citation17]. In the present study, cumulative doses of cisplatin refer both to initial treatment and any salvage therapy.
Overall, four patients received carboplatin as the only Pt agent and one received both carboplatin and cisplatin. For these men the corresponding cisplatin-equivalent doses were calculated by dividing carboplatin doses by four [Citation18].
The 94 TCSs were allocated to three different groups according to therapy: surgery only (controls) or cumulative cisplatin dose ≤850 mg (cis ≤850 group) or >850 mg (cis >850 group).
Assessments and definitions
Pt levels in serum samples from both surveys were quantified at St. Olav’s University Hospital in Trondheim, by inductively coupled plasma mass spectrometry (ICP-MS) [Citation7]. Pt concentrations were analyzed in batches for SI and SII, respectively. The levels of quantification (LOQ), calculated for SI and SII separately, were 13 and 15 ng/L in SI and SII, respectively. Pt concentrations measured below LOQ had values set to zero. Pt change was defined as [Pt (ng/L) at SI minus Pt (ng/l) at SII/years from SI to SII]. As most men had a decrease in the Pt level between SI and SII, this variable is hereafter termed Pt decline.
Information about second cancers after SII was retrieved from the Norwegian Cancer Registry in 2017 (cancer diagnosis status update 31 December 2015). One participant diagnosed with a second cancer (malignant melanoma in 1998) prior to SI was excluded from second cancer analyses.
The questionnaires at SI and SII included a validated scale for chemotherapy-induced neurotoxicity (SCIN) addressing neuropathy and Raynaud-like phenomena in hands and feet, tinnitus and impaired hearing [Citation19]. Each question was categorized according to symptom bother as 0, ‘not at all’; 1, ‘a little’; 2, ‘quite a bit’; or 3, ‘very much’. [Citation8]. At SI, eight TCSs had missing data for one or more NTX symptoms. TCSs were allocated into three different categories according to decreased, stable or increased symptom intensity in each of the NTX symptoms from SI to SII (NTX change; Supplementary Table 1).
Renal function was dichotomized at a serum creatinine level of 90 µmol/L [Citation14]. Smoking status as reported in the questionnaires was for ordinal regression models categorized into four groups based on responses in both SI and SII: ‘never smoker’, ‘previous smoker’ or ‘current smoker’ in both surveys, and ‘stopped smoking between SI and SII’. Stable current smokers served as a reference group. For Cox regression models, smoking was defined as current, previous or never smoker at SII, with never smokers serving as reference.
Physical activity was retrieved from the questionnaire at SII and categorized in-line with previous publications [Citation20,Citation21].
Statistical methods
Continuous variables were described as median and range. Categorical variables were described with counts and proportions. Correlations between continuous variables were assessed by Spearman’s rank correlation. The χ2 test was used to test associations between categorical distributions. The distribution of Pt levels was not normally distributed, and the Mann-Whitney U test was used to compare median values of Pt or Pt decline across different groups.
Cox proportional hazard regression models were used to estimate hazard ratios of a second cancer diagnosis after SII. The observation time ranged from the date of orchiectomy until the date of diagnosis of a second cancer or until 31 December 2015 for censored cases. The Cox regression models were also analyzed with an observation time that ranged from the date of SII, yielding the same results as the observation time that ranged from the date of orchiectomy. We have chosen to present results of analyses including the observation time ranging from the date of orchiectomy as this is considered to be more clinically relevant. Due to a low number of events, only two variables were included in the model [Citation22].
Associations between Pt decline and cisplatin dose as the explanatory factors and each SCIN symptom in SII or with NTX change from SI to SII as dependent variables were analyzed using multivariable ordinal logistic regression models, with scores of each SCIN symptom at SII in four categories, and three categories of NTX change, as previously described.
Model assumptions in the ordinal logistic regression models were assessed by the test of parallel lines (four out of 42 of our ordinal analyses had significant tests of parallel lines), whereas visual inspection of log minus log survival curves was used for model assumption in Cox regression models. All tests were two-sided and statistical significance was set at 0.05. Statistical analyses were carried out using IBM SPSS version 24 (IBM, Chicago, IL).
Results
Characteristics of patients
Median time from SI to SII was 8.5 years (6.7–9.3, ). The 77 cases treated with CBCT received median cumulative cisplatin dose 800 mg (range 178–3095), and 81% had 3 to 4 cycles. BEP and CVB were the most prevalent regimens administered (78%). Eight of the 94 TCSs received additional radiotherapy (7 dogleg, 1 para-aortal; dose-range 30–40 Gy).
Table 1. Details on demographics, treatment and platinum levels.
Pt levels and changes in Pt levels from SI to SII
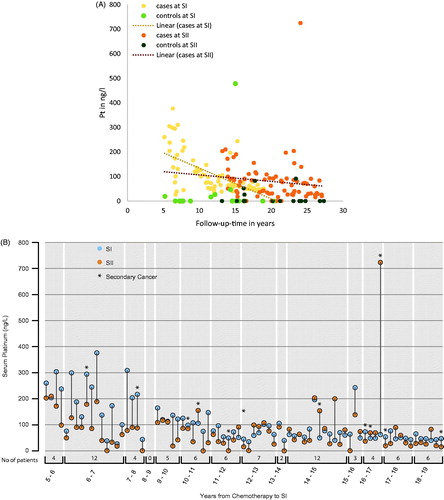
The median Pt level for all cases was 75 ng/L (0–377) and 64 ng/L (0–725) at SI and SII, respectively (). The median Pt level for the controls was 0 ng/L (0–478) at SI and 0 ng/L (0–91) at SII. The median Pt decline was significantly higher among cases than controls (4.2 vs. 0.0 ng/L/year, p = .005), and among men in the cis >850 mg group versus the cis ≤850 mg group (7.9 vs. 3.0 ng/L/year, p = .003) (). illustrate the change in Pt levels for the TCSs according to treatment and follow-up time, with higher Pt values for men with shorter follow-up time. In the Pt values of controls are also included, illustrating that some men had high Pt levels without prior CBCT. In , most men had declining Pt levels between SI and SII; however, 11 (14%) cases have significantly increasing Pt levels from SI to SII. For cases the cumulative cisplatin dose correlated significantly with a Pt decline from SI to SII (r = 0.30, p = .01).
Figure 2. (A) Pt levels at SI and SII for 94 participants, illustrated according to treatment modality and follow-up in years. Pt: platinum; cases: treated with chemotherapy; controls: treated with surgery only. (B) Pt levels at SI (blue dots) and SII (orange dots) for 77 CBCT-treated TCS, arranged according to follow-up time at SI. Pt: serum platinum; SI: survey I (1998–2002); SII: survey II (2007–2008); CBCT: platinum-based chemotherapy; TCS: testicular cancer survivors.
Pt levels and second cancers
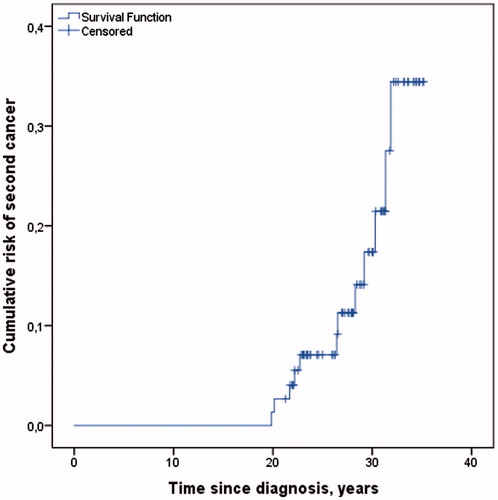
Among the 76 eligible cases, 12 men (15%) were diagnosed with a second cancer after SII. Median time from the TC diagnosis to the second cancer diagnosis was 26.5 years (range 19.9–31.9) (), and median time from SII to the second cancer diagnosis was 5.1 years (range 1.2–8.0). The second cancers included lung cancer, N = 2; malignant melanoma, N = 2; bladder cancer, N = 2; gastric cancer, N = 2; other GI cancers; N = 2; prostate cancer, N = 1 and head and neck cancer, N = 1.
Figure 3. Cumulative risk of second cancer among all 76 men included in the analysis, and time to the second cancer diagnosis among 12 men diagnosed with second cancer after Survey II.
Median Pt level decreased from SI to SII for men without a second cancer diagnosis (median 65.1 vs. 41.8 ng/L, p < .001), and median Pt level increased from SI to SII for men diagnosed with a second malignancy after SII (median 51.7 vs. 78.1 ng/L, p =.03).
A higher Pt level at SII was significantly associated with an increased risk for a second cancer diagnosis (HR 1.22, 95% CI 1.05–1.42 per 50 ng/L increase in Pt), whereas a larger decline of Pt from SI to SII was associated with decreased risk of a second cancer diagnosis (HR 0.78, 95% CI 0.62–0.99 per 10 ng/L/year) (). Current smokers at SII had increased risk of a second cancer diagnosis compared with never smokers (HR 9.14, 95% CI 1.88–45.0). The associations between Pt decline, smoking status and second cancer were stronger in the multivariable model including both variables ().
Table 2. Hazard ratios of second cancer diagnosed after survey II.
Pt decline, renal function and treatment
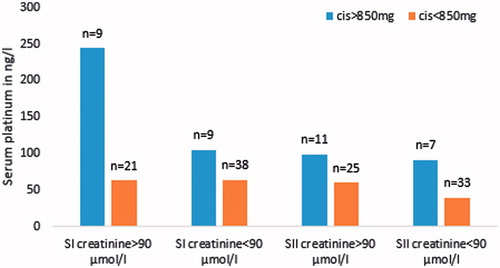
The cumulative cisplatin dose correlated significantly with creatinine level at SI and SII (r = 0.25, p = .03 and r = 0.24, p = .04, respectively). There were significant correlations between Pt at SI and creatinine at SI (r = 0.50, p = .03). Overall, Pt values were higher among cases with cis >850 with creatinine >90 µmol/L in SI (p = .05) ().
Pt decline and neuro- and ototoxicities
At least one SCIN symptom was reported by 49 (missing, n = 8) (64%) and 69 (90%) men at SI and SII, respectively. All SCIN symptoms at SII were significantly worsened compared with SI (p < .001 for all, Supplementary Table 1). Analyses showed crude associations between Pt decline from SI to SII and paresthesias in hands (r= 0.23, p = .05) and feet (r = 0.25, p = .03) at SII.
In age-adjusted ordinal regression analyses, men with a larger Pt decline from SI to SII had significantly higher risks of increasing symptom intensity from SI to SII of paresthesias in hands (OR 1.98, 95% CI 1.09–3.59, per 10 ng/L/year) and tinnitus (OR 1.51, 95% CI 1.01–2.27, per 10 ng/L/year). These findings remained significant when adjusted for cisplatin dose ().
Table 3. Odds ratios of increased symptoms between SI to SII of paresthesias in hands and feet, Raynaud’s in hands and feet, tinnitus and hearing impairment using ordinal logistic regression models*.
Patients who quit smoking between SI and SII had a lower risk of experiencing deterioration of Raynaud’s symptoms in hands (OR 0.09, 95% CI 0.01–0.59) and feet (OR 0.12, 95% CI 0.02–0.69) compared with current smokers. Those who never smoked had a lower risk of deteriorating Raynaud’s symptoms in feet (OR 0.15, 95% CI 0.04–0.60).
In ordinal regression analyses, Pt decline was not associated with any SCIN symptoms at SII (Supplementary Table 2). The risk of higher symptom intensity at SII increased with cumulative cisplatin dose (per 100 mg) for all SCIN symptoms except Raynaud’s symptom in hands (Supplementary Table 2).
Discussion
To the best of our knowledge, this is the first study demonstrating long-term Pt change for survivors followed up to 27 years after CBCT. Importantly, a larger Pt decline from SI to SII was associated with a decreased risk for a second cancer, but with worsening of paresthesias in hands and tinnitus.
The major strength of this study comprises a well-described cohort of TCSs with extensive data regarding CBCT cancer therapy. All men underwent standardized examinations, and measurements of Pt levels were available at both SI and SII, allowing us to present the longest follow-up for serum Pt change published to date. Our study originates from a large cohort of TCSs, but the numbers of patients available for Pt analyses were limited, hence limiting statistical power, with the risk of underestimating associations. Our study does not include neurological tests, but presents self-reported SCIN, representing a clinical relevant measure, addressing the extent to which symptoms affect quality of life. Furthermore, multiple testing increases the probability of false-positive tests only due to chance. The Bonferroni correction test is considered conservative, to the extent that true associations may remain undetected. In exploratory and epidemiological studies where a final conclusion might not be necessary nor possible, corrections are not considered compulsory [Citation23,Citation24].
Pt elimination rates could only be estimated in a linear manner, as our study included Pt measurements at two time points per participant only. Obtaining Pt measurements at numerous time points would have improved the quality of Pt elimination analyses. Boer et al. [Citation14] have presented a pharmacokinetic model which included Pt concentrations, urinary excretion rates, cisplatin dose, age, weight and height from 98 patients. On the basis of this model they used the area under the curve one to three years after chemotherapy as a Pt exposure variable [Citation14], and this value was significantly associated with cumulative cisplatin exposure, paresthesias, CVD risk factors and hypogonadism nine years after treatment. However, their analyses were not adjusted for cumulative cisplatin doses, which could have clarified whether the exposure between one and three years merely reflects the treatment burden. Hence, it is still unknown if the cisplatin dose and the long-term Pt exposure are separate mechanistic factors in the development of late effects. We need a better understanding of long-term Pt pharmacokinetics to elucidate this issue.
The mechanism behind a Pt increase from SI to SII for some TCSs remains unknown. Pt released from compartments with separate pharmacokinetics may be a possible explanation. Pt residuals can be found in fat tissue, thyroid gland and a range of organs such as liver, kidney, bone and lungs after chemotherapy [Citation25]. The principal environmental contamination of Pt, a metal of the platinum group elements (PGE), comes from catalytic car converters. During the last decades, the concentration of Pt in environmental samples, such as soil, surface water and plants has significantly increased [Citation26–29]. Biomonitoring studies have shown that the body-burden levels of PGE among urban populations and those working in close proximity with traffic, reflects their higher exposure to these metals [Citation26]. Moreover, a German study found Pt concentrations in airborne particulate matter to be 6 times higher for samples collected in 2008–2010 compared with 2002 [Citation30]. Therefore, exposure to other possible Pt compounds may explain the increasing Pt levels measured in some of the men in our cohort. Of note, cisplatin is the only known Pt compound that is carcinogenic [Citation31].
Second cancer is a leading cause of death among long-term TCSs after cytotoxic treatment [Citation3]. Several publications have demonstrated elevated relative risks for solid second cancers after CBCT in most follow-up periods, but particularly with long follow-up beyond 20 years [Citation32–34]. Although the number of second cancers observed in our study is too low to make relative risk calculations, the cancer sites registered in our cohort corroborate data from the Danish study reporting increased risks for cancers in the lung and bladder, among others [Citation32]. To our knowledge, the present study is the first to demonstrate a relationship between a larger decline in Pt levels and a reduced risk of having a second cancer, whereas cumulative cisplatin dose was not associated with the risk of second cancer. Thus, a better understanding of long-term Pt storage and pharmacokinetics seems to be important to clarify mechanisms for development of second cancers after CBCT.
Decreased renal function after TC treatment is closely related to an increased number of BEP cycles [Citation35]. Boer and coworkers [Citation14] conclude that renal function, both prior to and shortly after treatment, is a strong determinant of long-term exposure to circulating Pt. Herein, we demonstrate a significant association between Pt levels at SI and creatinine >90 µmol/L at SI. The lack of an association between Pt decline and creatinine levels could possibly be due to a limited study population.
We have previously demonstrated that associations between long-term Pt levels and most NTX symptoms diminish over time, whereas cumulative administered cisplatin doses remain associated with NTX [Citation8,Citation21]. The present study shows that a larger Pt decline correlated positively with higher cisplatin doses, and was associated with a worsening of paresthesias in hands and tinnitus from SI to SII. As cisplatin-induced peripheral neuropathy (CIPN) is a typical long-term effect developing during or shortly after treatment [Citation36], it is probably more likely associated with the cumulative administered cisplatin dose than the long-term Pt decline. The exact pathogenesis of long-term CIPN is largely unknown, but relatively high-Pt levels have been found in the dorsal roots in a postmortem study [Citation37]. To which extent cisplatin dose corresponds with Pt levels in the dorsal roots is however hitherto unknown.
In a recent paper investigating postmortem long-term cisplatin retention in cochlea and its relationship with hearing impairment, high-Pt levels were found in cochlea and long bones [Citation38]. The hypothesis that bone may serve as a reservoir for Pt, leading to Pt hyper-accumulation and long-term destruction of cochlear and bone cells, is supported by the demonstration of extensive Pt binding to, and slow dissociation from, type 1 collagen, the major protein component of bone [Citation39]. In addition, Pt pharmacokinetics are comparable with those of lead, a heavy metal that distribute into bone and can be exchanged back into the blood, with a half-life of years-to-decades [Citation40]. Whether or not prolonged release of Pt from bone mediates late toxicities needs further investigation. Especially, Pt stored in vertebras might be useful to assess due to their proximity to the nervous system and dorsal roots.
Smoking is a major risk factor for several cancers [Citation41–43]. Herein, current smokers at SII had an increased risk of a second cancer diagnosis compared with never smokers. Never smokers and men who quit smoking between SI and SII had a lower risk of increasing Raynaud’s symptoms, corroborating smoking as an independent risk factor for Raynaud’s phenomenon [Citation44]. Hence, smoking cessation should be a priority during long-term follow-up of TCSs.
In summary, we found an association between a larger Pt decline and a reduced risk of second cancers, and deterioration of paresthesias in hands and tinnitus in TCSs. Hypothetically, different mechanisms are involved according to whether the cisplatin-induced side effect occurs during or shortly after chemotherapy, as with NTX, or several years after treatment, as with second cancers. Associations between retained Pt and second cancers need to be further investigated in larger studies.
Seung_Eun_Jung_et_al._Supplementary_tables.zip
Download Zip (23.7 KB)Acknowledgments
The authors thank project secretaries Vigdis Opperud and Siri Lothe. The study is a national clinical study as part of the Norwegian Urological Cancer Group III project.
Disclosure statement
S. D. Fosså has received funding from Janssen Norway and Astellas Norway, and has an advisory role for Janssen Norway and Astellas Norway. There are no other author disclosures.
References
- Cancer in Norway 2012 – Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2014.
- Aziz NM. Cancer survivorship research: state of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–432.
- Haugnes HS, Bosl GJ, Boer H, et al. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J Clin Oncol. 2012;30:3752–3763.
- Haugnes HS, Stenklev NC, Brydoy M, et al. Hearing loss before and after cisplatin-based chemotherapy in testicular cancer survivors: a longitudinal study. Acta Oncol. 2018[cited 2018 Jan 31]; [9 p.]. DOI:10.1080/0284186X.2018.1433323
- Oldenburg J, Kraggerud SM, Cvancarova M, et al. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714.
- Gietema JA, Meinardi MT, Messerschmidt J, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet. 2000;355:1075–1076.
- Hjelle LV, Gundersen PO, Oldenburg J, et al. Long-term Platinum retention after platinum-based chemotherapy in testicular cancer survivors: a 20-year follow-up study. Anticancer Res. 2015;35:1619–1625.
- Sprauten M, Darrah TH, Peterson DR, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in cisplatin-treated survivors of testicular cancer. J Clin Oncol. 2012;30:300–307.
- Hohnloser JH, Schierl R, Hasford B, et al. Cisplatin based chemotherapy in testicular cancer patients: long term platinum excretion and clinical effects. Eur J Med Res. 1996;1:509–514.
- Brouwers EE, Huitema AD, Beijnen JH, et al. Long-term platinum retention after treatment with cisplatin and oxaliplatin. BMC Clin Pharmacol. 2008;8:7.
- Schierl R, Rohrer B, Hohnloser J. Long-term platinum excretion in patients treated with cisplatin. Cancer Chemother Pharmacol. 1995;36:75–78.
- Gelevert T, Messerschmidt J, Meinardi MT, et al. Adsorptive voltametry to determine platinum levels in plasma from testicular cancer patients treated with cisplatin. Ther Drug Monit. 2001;23:169–173.
- Oldenburg J, Kraggerud SM, Brydoy M, et al. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70.
- Boer H, Proost JH, Nuver J, et al. Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol. 2015;26:2305–2310.
- Brydoy M, Oldenburg J, Klepp O, et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 2009;101:1682–1695.
- Peckham MJ, McElwain TJ, Barrett A, et al. Combined management of malignant teratoma of the testis. Lancet. 1979;2:267–270.
- Brydoy M, Fossa SD, Klepp O, et al. Paternity and testicular function among testicular cancer survivors treated with two to four cycles of cisplatin-based chemotherapy. Eur Urol. 2010;58:134–140.
- Ozols RF, Behrens BC, Ostchega Y, et al. High dose cisplatin and high dose carboplatin in refractory ovarian cancer. Cancer Treat Rev. 1985;12:59–65.
- Oldenburg J, Fossa SD, Dahl AA. Scale for chemotherapy-induced long-term neurotoxicity (SCIN): psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res. 2006;15:791–800.
- Thorsen L, Nystad W, Dahl O, et al. The level of physical activity in long-term survivors of testicular cancer. Eur J Cancer. 2003;39:1216–1221.
- Hjelle LV, Bremnes RM, Gundersen PO, et al. Associations between long-term serum platinum and neurotoxicity and ototoxicity, endocrine gonadal function, and cardiovascular disease in testicular cancer survivors. Urol Oncol. 2016;34:487.e13–e20.
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718.
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170.
- Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54:343–349.
- Dikhoff TGMH, Goeij JJM, Mcvie JG. Long-term body retention and tissue distribution of platinum in cisplatin treated cancer patients. J Radioanal Nucl Chem. 1998;236:81–86.
- Caroli S, Alimonti A, Petrucci F, et al. Assessment of exposure to platinum-group metals in urban children. Spectrochim Acta B. 2001;56:1241–1248.
- Morton O, Puchelt H, Hernández E, et al. Traffic-related platinum group elements (PGE) in soils from Mexico City. J Geochem Explor. 2001;72:223–227.
- Pawlak J, Lodyga-Chruscinska E, Chrustowicz J. Fate of platinum metals in the environment. J Trace Elem Med Biol. 2014;28:247–254.
- Barbante C, Veysseyre A, Ferrari C, et al. Greenland snow evidence of large scale atmospheric contamination for platinum, palladium, and rhodium. Environ Sci Technol. 2001;35:835–839.
- Wiseman CLS. Platinum metals in airborne particulate matter and their bioaccessibility. In: Zereini F, Wiseman CLS, editors. Platinum metals in the environment. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. p. 447–462.
- IARC Monographs [Internet]. Lyon, France:International Agency for Research on Cancer;1987. Available from: http://monographs.iarc.fr/ENG/Monographs/suppl7/Suppl7-53.pdf.
- Kier MG, Hansen MK, Lauritsen J, et al. Second malignant neoplasms and cause of death in patients with germ cell cancer: a Danish Nationwide Cohort Study. JAMA Oncol. 2016;2:1624–1627.
- Fung C, Fossa SD, Milano MT, et al. Solid tumors after chemotherapy or surgery for testicular nonseminoma: a population-based study. J Clin Oncol. 2013;31:3807–3814.
- Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365.
- Lauritsen J, Mortensen MS, Kier MG, et al. Renal impairment and late toxicity in germ-cell cancer survivors. Ann Oncol. 2015;26:173–178.
- Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14:iv45–iv54.
- Krarup-Hansen A, Rietz B, Krarup C, et al. Histology and platinum content of sensory ganglia and sural nerves in patients treated with cisplatin and carboplatin: an autopsy study. Neuropathol Appl Neurobiol. 1999;25:29–40.
- Breglio AM, Rusheen AE, Shide ED, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8:1654.
- Chang Q, Ornatsky OI, Siddiqui I, et al. Biodistribution of cisplatin revealed by imaging mass cytometry identifies extensive collagen binding in tumor and normal tissues. Sci Rep. 2016;6:36641.
- Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106:1.
- Islami F, Moreira DM, Boffetta P, et al. A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur Urol. 2014;66:1054–1064.
- Bjerkaas E, Parajuli R, Weiderpass E, et al. Smoking duration before first childbirth: an emerging risk factor for breast cancer? Results from 302,865 Norwegian women. Cancer Causes Control. 2013;24:1347–1356.
- Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23:1880–1888.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:e006389.