Abstract
Introduction: Parental socioeconomic status has been proposed to have an influence on childhood cancer mortality even in high-income countries. Our study investigated the influence of parental socioeconomic factors on childhood cancer mortality.
Material and methods: We identified 4437 patients diagnosed with cancer under the age of 20 from 1990 to 2009 and their parents from the Finnish cancer and central population registers. Information on death from primary cancer during five-year follow-up and parental socioeconomic factors was obtained from Statistics Finland. Poisson regression modeling was used to estimate hazard ratios (HRs) for factors related to cause-specific mortality and recursive tree based survival analysis to identify important risk factors and interactions.
Results: Mortality was lower in the highest quartile of combined parental disposable income (HR 0.68, CI 95% 0.52–0.89) compared to the lowest quartile. In the most recent diagnostic period from 2000 to 2009, highest attained education of either parent being post-secondary predicted lower mortality (HR 0.73, CI 95% 0.60–0.88) compared to parents who had attained primary or lower education.
Conclusion: Despite high quality public health care and comprehensive social security, both high parental income and education were associated with lower mortality after childhood cancer. Lower health literacy and financial pressures limiting treatment adherence may explain higher mortality in children with less educated parents and parents with lower income. Motivation and support during treatment and follow-up period is needed concerning the families of these patients.
Introduction
Childhood cancer survival has improved dramatically over the past four decades in high-income countries due to improvement in diagnostics and treatment [Citation1]. In Finland, overall five-year childhood cancer survival reached 80.9% in 2000 with no significant improvement thereafter [Citation2,Citation3]. There is thus a growing need to explore possible other than treatment related factors influencing childhood cancer survival.
Low socioeconomic status has been shown to contribute to worse survival of childhood cancer in low-, middle- and as well as high-income countries [Citation4]. Nordic studies exploring the effect of parental socioeconomic status and family factors on childhood cancer survival and mortality have suggested that lower parental education level [Citation5,Citation6], single parenthood [Citation7] and having siblings [Citation6,Citation7] result in decreased survival after childhood cancer.
Education in Finland is free of charge and mostly government funded. In 2016, the educational level of the population over 15 years of age was primary education or less in 28%, secondary education in 38% and post-secondary education in 34% [Citation8]. The median disposable household income per year was 31,700 EUR [Citation9] and the average unemployment rate was 8.8% that year [Citation10]. The socioeconomic circumstances in Finland are comparable with other Nordic countries, in which the public authorities guarantee adequate and equal provision of social, medical and health services for everyone [Citation11]. The public health care system consists of general practitioner led primary health care and preventive services in health centers. Specialized secondary and tertiary medical care in hospitals is accessed by referral mainly via primary care. Though the majority of health care service providers and hospitals in Finland are public, there is also an opportunity to seek medical care in the private sector. Childhood cancer treatment is centralized to five public sector tertiary level university hospitals, located in Helsinki, Kuopio, Oulu, Tampere and Turku, and is uniform throughout the country. A majority of childhood cancer patients are treated in the Helsinki area (32%) which has the highest population density. By surface area, the largest but based on population size smallest, is the Oulu region in the north of Finland (16% of treated cancers), with a university hospital providing tertiary level healthcare. The aforementioned factors could be expected to minimize the impact of socioeconomic factors on childhood cancer survival.
The aim of this population based study was to investigate the associations between parental socioeconomic status, family characteristics and childhood cancer mortality. We utilized information on both parents and as novelty we investigated the impact of parental employment status on childhood cancer mortality.
Material and methods
Registers
The Finnish Cancer Registry (FCR), established in 1952, is nation-wide and population-based and covers the population since 1953. Health care personnel are obliged to report cancer cases to the FCR and the quality of the registry is high with 95% overall coverage [Citation12]. The registry data includes details of primary site, histology, extent of disease, time of diagnosis and primary treatment (yes/no basis). Cancers are coded using to the International Classification of Diseases for Oncology (ICDO)-3 coding system and following the International Agency for Research on Cancer (IARC)/ International Association for Cryptologic Research (IACR) coding rules [Citation13] and it’s available for the whole patient cohort. The older cancer coding has been converted to ICDO-3.
Our study population consisted of childhood cancer patients aged under 20 years at diagnosis occurring between 1990 and 2009, identified from the FCR. Parents and siblings of childhood cancer survivors were identified from the central population registry held by the Population Register Center (PRC) founded in 1969. The patients were followed for death or emigration using the PRC. Each Finnish citizen and permanent resident living in Finland is given a unique personal identity code which enables linkage between the different health registries and databases.
Statistics Finland produces statistics and data describing various socioeconomic factors in the population and the different socioeconomic variables are mostly available annually from 1987 onwards. Information on all parental socioeconomic factors and on the cause of death of the deceased patients was retrieved from Statistics Finland.
Socioeconomic information
Information on individual disposable income [Citation9] has been divided into 10 categories annually from 1995 on by Statistics Finland. Data on the disposable income of both parents was retrieved and combined parental disposable income was calculated. Based on the aforementioned categories parental income was further divided into quartiles after comparing the income preceding the year of diagnosis annually to national income statistics. Due to the restricted availability of the variable, information on income was missing for 1338 patients (patients diagnosed before 1996). These patients were analyzed in a separate category “structural missing”. With patients diagnosed in 1996 and thereafter, the category was “information missing” if the information was not available from both parents.
Information on the highest attained education for both parents was retrieved from the Register of Completed Education and Degrees [Citation8] and categorized into three levels: Primary education or less, secondary education, and post-secondary education. Secondary education stands for high-school or vocational training. Post-secondary education includes university education, polytechnic education and other post-secondary education. The Register of Completed Education and Degrees includes information on secondary and further education. Thus, information on completion of compulsory education was not available and therefore all missing values were included in the category of primary education or less.
Parental working ability was assessed by employment status [Citation14], which represents the principal activity at the end of the calendar year. Employment status was divided into five categories: Employed, unemployed, student, pensioner and other persons outside the labor force.
Information on co-habitation status [Citation15] for both biological and adopted parents was obtained and included in two categories: Cohabiting and single-parent. The link between biological parents could not be attained so the parent could be cohabiting with other than the child’s biological parent. The number of full- and half-siblings at the time of the patient’s cancer diagnosis was divided into five categories: An only child, one sibling, two siblings, three siblings and four or more siblings. Maternal age at the time of the child’s cancer diagnosis was classified into four categories: Under 26 years, 26–36 years, 36–45 years and over 45 years.
Statistical analyses
Poisson regression modeling using person-years as an offset term was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for cause-specific mortality, adjusting for age at cancer diagnosis and diagnostic period in the first model and adjusting for age at cancer diagnosis, diagnostic period, sex, cancer type, combined parental income, highest parental education, parental employment status and maternal age in the second model (fully adjusted model). The primary outcome was death from primary cancer. We divided the follow-up time into four calendar periods: 1990–1994, 1995–1999, 2000–2004 and 2005–2009. Age at cancer diagnosis was divided into four categories: Under 1 year, 1–9, 10–15 and 16–19 years. Primary diagnoses were grouped into three categories primarily based on the crude burden of treatment: Acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) [Citation16] (treatment duration from 2–2.5 years), central nervous system (CNS) tumors (high treatment burden and effects on the central nervous system) and all other malignant neoplasms (treatment usually lasting from a half a year to one year). Analyses were also performed separately by sex of the patient. We also compared whether treatment (based on data available in the FCR) was different between income categories and results of these models were corrected for multiple comparisons using the Bonferroni correction, which is known to be conservative.
Analyses for combined parental income and highest parental education were conducted choosing either the maternal or paternal educational level, whichever was highest. Employment status and parental co-habitation was analyzed for both parents separately. Missing values were included in the analyses as a separate category. Information on parental socioeconomic variables was retrieved from the year preceding the child’s diagnosis. Analyses for maternal age and number of siblings was conducted based on the information at the time of child’s cancer diagnosis. Follow-up started at diagnosis of cancer and ended at death from primary cancer, emigration or 31 December 2014, whichever occurred earliest. The analyses for mortality and survival were conducted both for a maximum of five years of follow-up. Deaths due to causes other than primary cancer (n = 37, 4.6% of all deaths) were censored. Childhood cancer specific survival was estimated using the life-table method. Differences between survival curves were tested for using log-rank test. Classification and regression trees (CART) with the recursive partition method were applied to explore factors related to survival/mortality [Citation17–19]. This was done because we had no preconception which factors or their combinations that would be relevant for childhood cancer mortality. Despite the relatively large sample size, the data can become sparse as death is a rare event and due to interactive effects. In such cases interactions between variables are difficult to detect with traditional statistical methods and the CART method has better performance. Further in the recursive partition classification tree we do not have to assume specific functional dose-response allowing flexible form. All statistical analyses were performed using the R version 3.2.2, packages popEpi (ver 0.4.1;2015) and party (ver 1.2–2).
Ethical considerations
This study was based on register data and ethical approval was included in the permission process. Permits for registry linkage were obtained from the National Institute for Health and Welfare (THL/970/5.05.00/2010, THL/520/5.05.00/2016), Population Register Center (VRK854/410/16) and Statistics Finland (TK53-358-16).
Results
Patient characteristics
In all, 4437 childhood cancer patients were identified from the Finnish Cancer Registry from 1990 to 2009 (). There was a slight male predominance with 53% (n = 2358) of patients being boys and 47% (n = 2079) girls. The majority of patients had been diagnosed with cancer at 1–9 years of age (42%). The distribution of primary diagnosis was similar to that described worldwide, with 21% being diagnosed with ALL or LBL, 21% with CNS tumors and 58% with other malignant neoplasms. The distribution of cancer diagnoses by age and International Classification of Childhood Cancer (ICCC)-3 categories as well as the categorization of cancer types is presented in the Supplementary Table S1.
Table 1. Characteristics of the children diagnosed with cancer at the age of 0–19 years in 1990–2009.
Identification of both parents was possible in 98% of patients, leaving 68 patients for whom information on the father was missing and seven patients, for whom information on both parents was missing. These patients were included in all analysis but were in the category ‘information missing’ if parental information was not available.
Mortality and survival
Seven hundred sixty nine deaths from primary cancer occurred during 19,318 person-years of follow-up. We found cancer mortality overall to be lower in girls compared to boys (HR 0.82, CI 95% 0.71–0.95; ). By age at diagnosis, the poorest survival was seen in children aged less than one year at diagnosis. Survival of childhood cancer patients improved from the beginning of 1990 to the turn of the century.
Table 2. Five-year cancer mortality for all patients in two adjusted models.
Overall, cancer mortality was lowest in the highest combined parental income quartile compared to the lowest quartile (HR 0.68, CI 95% 0.52–0.89) when adjusting for age and diagnosis and diagnostic period (). After further adjustment for parental education, mortality was still significantly decreased in the highest parental income category compared to the lowest (Q2 HR 0.83, CI 95% 0.64–1.10; Q3 HR 0.78, CI 95% 0.59–1.03; Q4 HR 0.72, CI 95% 0.55–0.95). In the fully adjusted model adjusting additionally for parental employment status, mother’s age, sex and cancer type the results remained significant. When analyzing mortality by cancer type and adjusting for age at diagnosis and diagnostic period, lower mortality in the highest combined income quartile compared to the lowest one (HR 0.62, CI 95% 0.43–0.90) was seen in the category of patients with other malignant neoplasms (). The results regarding ALL, LBL and CNS tumors were not statistically significant, but point estimates suggested similar patterns where mortality was lower if parents were in the highest income quartile.
Table 3. Five-year cancer mortality for different cancer types adjusted for age at diagnosisa and diagnostic periodb.
In the overall analysis, mortality was lower in patients with more educated parents, but the difference was not statistically significant. Using regression tree modeling, we found that mortality, adjusted for age at diagnosis, was decreased in the latest diagnostic periods if parental educational level was post-secondary compared to secondary or less (HR 0.66, CI 95% 0.54–0.81; ) using the patients diagnosed in the 1990’s with parental education being primary or secondary as a reference group. Mortality was similar in patients diagnosed in the 1990’s regardless of parental education and in patients diagnosed between 2000 and 2009 with parental education being primary or secondary. The groups were further subdivided by maternal cohabitation status. We saw an increasing trend in mortality of patients diagnosed between 2000 and 2009 with less educated single mothers. The significant mortality difference in this time period was lowest in cohabiting mothers with education being post-secondary.
Table 4. Effect of diagnostic period, education and maternal co--habitation on five-year cancer mortality and five-year survival.
When comparing to employed parents, there were no significant differences in cancer mortality if parents were unemployed, students, pensioners or not working for some other reason in the model adjusted for age at diagnosis and diagnostic period. However, in the fully adjusted model adjusting additionally for parental income, education, mother’s age, sex and cancer type, mortality was lower if the mother was unemployed (HR 0.75, CI 95% 0.57–0.99) compared to a mother being employed.
In the overall analyses, parental cohabitation (living alone or living with a spouse), mother’s age or the number of siblings at the time of the child’s diagnosis were not associated with mortality in our study (data for parental cohabitation and the number of siblings not shown).
Combined parental income significantly influenced five-year cause specific cancer survival (p = .003). Survival was 86% (83–88%) in the highest income quartile and 79% (75–82%) in the lowest income quartile. There were no significant differences in survival by parental education (p = .06) though the results suggest higher survival with higher parental education. When using the regression tree modeling, we found that patients diagnosed in the 1990’s had similar survival regardless of parental education but patients diagnosed in the most recent time periods had a significantly higher survival if parental education was post-secondary ( and ). In the latest diagnostic periods from 2000 to 2009, lower parental educational level and being a single mother decreased survival (, ).
Figure 1. The Regression-tree model and five-year cancer survival. A) Period effect and the effect of parental education. B) Period effect and the effect of parental education and maternal co-habitation.
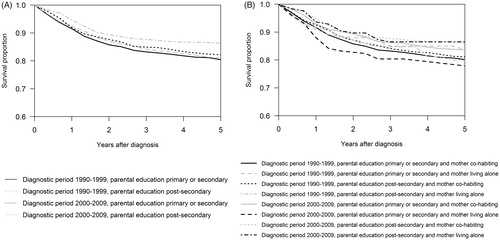
Analyses of the socioeconomic factors were performed for both female and male patients separately, but the results by sex specific analyses did not differ significantly from models with both sexes combined.
Treatment data
Comparing received treatment (surgical treatment, radiation therapy, chemotherapy, hormonal treatment and other treatment) in different income categories, we did not find statistically significant differences after Bonferroni correction (data not shown).
Discussion
In this study we found that parental income was inversely associated with childhood cancer mortality. Mortality was higher for the children of less educated parents and for children living with less educated single mothers, between 2000 and 2009. There were no differences in mortality when analyzing the socioeconomic factors by sex of the patient as we expected.
Year 1990 was selected as the beginning of follow-up since it marked the start of a more uniform protocol-based treatment era in most of childhood cancer types. Also the limited availability of many socioeconomic variables restrict the use of older data. The categories by age at diagnosis were made based on differences in treatment, cancer types and prognosis at different ages. Cancer types were categorized into three main types based on treatment burden. We hypothesized that high treatment burden could contribute to the observed high mortality in the case of patients with lower parental socioeconomic status. However, we found no clear differences in mortality between cancer types. A recent Danish study [Citation7] suggested that maternal income may affect childhood cancer survival (fully adjusted HR 0.84, CI 95% 0.66–1.08), but both a Swedish and a Norwegian study on childhood cancer [Citation5,Citation6] found no influence of parental income on mortality. In a study analyzing overall child mortality in Finland from 1990 to 2004 [Citation20], low parental income was associated with higher overall mortality. Since pediatric oncological treatment is offered solely in the public sector, parental income is unlikely to influence provision of cancer treatment in the hospital setting. Likewise, our findings on treatment data also imply that there are no differences in the given treatment based on parental income. Parents with higher income may have private health insurance for their children offering the opportunity to seek primary care diagnostics and treatment in the private sector where access to healthcare may be obtained faster and more easily. Therefore, the child’s presenting symptoms may be noticed and examined earlier and the child is sent to the hospital for specialized medical care at an earlier stage of the cancer. Higher parental income is also prone to reduce the financial burden after diagnosis when staying at home with the sick child and may give more mental resources to the parents with less stress from lost income. Though the health care system and social circumstances are quite comparable in the Nordic countries, there are differences in income distribution which make comparison difficult and can explain the differences in the results between the respective countries [Citation21]. Our results show that patients with other malignant neoplasms had lower mortality if parental income was higher. The results in ALL, LBL and CNS tumor patients were not significant, but suggestive in the same direction. Sparsity of data is the most likely reason for not observing significant differences in mortality in these diagnostic groups.
Higher parental education has been shown to be a predictor of lower mortality in childhood cancer compared to those with lower parental education [Citation5,Citation6,Citation22]. In our study overall, parental education did not appear to significantly influence mortality in the analysis including all patients independent of diagnostic era. Using the regression tree model we found that in the recent diagnostic period from 2000 to 2009, patients with one or both parents having post-secondary education had higher survival compared to patients with parental education being basic or secondary. Low parental education may equate to lower health literacy [Citation23,Citation24]. Thus, more educated parents may have more knowledge and understanding of the symptoms of the child and may seek treatment earlier in the first place and after diagnosis may be more likely to notice infection during therapy and recurrence thereafter. Treatment compliance may also be higher [Citation25]. It is interesting that the association between education and mortality is more clearly seen in the most recent diagnostic periods. When childhood cancer survival has increased significantly from the early nineties the gaps between socioeconomic groups seem to be getting wider. In the Finnish adult population, differences in health status between different socioeconomic groups have become more pronounced and are visible on a global level as well [Citation26,Citation27].
To our knowledge, this is the first study to explore the effects of parental employment status on childhood cancer mortality on an individual level. We found that parental employment status did not impact childhood cancer mortality in the model adjusted for age at diagnosis and diagnostic period. However, when adjusting for socioeconomic factors, mortality was lower if the mother was unemployed. When having a detailed look, combined parental income and parental education had the largest effect. If the socioeconomic situation of the family is secured, the unemployed mother can have more time and resources when taking care of the sick child.
Parental cohabitation status has been suggested to have an influence on childhood cancer mortality, better survival has been observed if parents were living together compared to living alone [Citation7]. The same effect has been seen in overall childhood mortality in Finland with children under 10 years of age [Citation20]. In our study there was a trend suggesting that patients diagnosed from 2000 to 2009 and with mothers as single parents and having low education (primary education or less) had higher mortality than patients with cohabiting mothers or patients with highly educated parents. Higher mortality among single parents may be due to the fact that parents living alone might not have the same resources as parents living with a spouse when you can share the burden with another adult.
The main strength of the study is using nationwide population based registry data concerning both cancer and various socioeconomic factors. As data on socioeconomic indicators were retrieved from national registries, they were free of reporting bias. There was little missing data with the exception of income. Due to restrictions in availability, 34% of the income data was missing (before 1995) limiting the data. In our registry data, we don’t have reliable information on staging or risk-classification in most of the childhood cancer cases, for example leukemias. As there was no information on stage at diagnosis, the relation between income and possible pre-diagnostic delay could not be examined. The information on socioeconomic and family factors was retrieved from the year preceding the child’s cancer diagnosis. This model has advantages since it eliminates the effect of the child’s cancer diagnosis on the family’s social and economic situation but also has limitations in not being able to detect the effect of cancer diagnosis on these aforementioned factors. We were able to compare received treatment based on different income categories on a highly aggregated level. Individual treatment protocols could not be included as an explanatory variable/risk factor due to evolution of protocols over the long follow up period within a single diagnostic group due to diversity of diagnoses within each disease category.
Our study indicates that even with availability of comprehensive public health care and social security along with government funded education, childhood cancer patients with less educated parents and parents with lower income had higher mortality compared to patients with higher parental education and income. High parental education likely translates to higher level of healthcare literacy possibly leading to higher commitment to treatment and higher sensitivity on reacting to child’s symptoms leading to care at an earlier stage. Lower mortality concerning children with higher parental income can be influenced by better mental and material resources of the parents with higher income due to having less stress from losing income. Motivation to treatment and follow-up and other support actions are important in the patient groups at risk for higher mortality.
Supplemental Material
Download MS Word (17.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gatta G, Botta L, Rossi S, et al . Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15:35–47.
- Cancer Society of Finland. Cancer in children and adolescents; 2016. [cited 2017 Aug 1]. Available from: https://www.cancersociety.fi/publications/reports/cancer-in-finland-2016/cancer-in-children-and-adolescents/
- Madanat-Harjuoja LM, Pokhrel A, Kivivuori SM, et al. Childhood cancer survival in Finland (1953-2010): a nation-wide population-based study. Int J Cancer. 2014;135:2129–2134.
- Gupta S, Wilejto M, Pole JD, et al. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review. PloS One. 2014;9:e89482.
- Mogensen H, Modig K, Tettamanti G, et al. Socioeconomic differences in cancer survival among Swedish children. Br J Cancer. 2016;114:118–124.
- Syse A, Lyngstad TH, Kravdal O. Is mortality after childhood cancer dependent on social or economic resources of parents? A population-based study. Int J Cancer. 2012;130:1870.
- Simony SB, Lund LW, Erdmann F, et al. Effect of socioeconomic position on survival after childhood cancer in Denmark. Acta Oncol. 2016;55:742–750.
- Statistics Finland. Official Statistics of Finland (OSF): students and qualifications of educational institutions; 2016. [cited 2017 Apr 28]. Available from: http://www.stat.fi/til/opiskt/index_en.html/
- Statistics Finland. Official Statistics of Finland (OSF): total statistics on income distribution. 2016 [cited 2017 Apr 28]. Available from: http://www.stat.fi/til/tjkt/index_en.html/
- Statistics Finland. Official Statistics of Finland (OSF): Labour force survey. 2016 [cited 2018 Jan 23]. Available from: http://www.stat.fi/til/tyti/2016/13/tyti_2016_13_2017-04-12_kat_002_en.html/
- Ministry of Social Affairs and Health. Health Care in Finland. 2013 [cited 2017 Apr 12]. Available from: http://julkaisut.valtioneuvosto.fi/handle/10024/69930
- Leinonen MK, Miettinen J, Heikkinen S, et al. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–39.
- Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology (ICD-O 3), 3rd ed. Geneva: World Health Organization; 2000.
- Statistics Finland. Official Statistics of Finland (OSF): Employment; 2016. [cited 2017 Apr 28]. Available from: http://www.stat.fi/til/tyokay/index_en.html/
- Statistics Finland. Official Statistics of Finland (OSF): Families; 2016. [cited 2017 Apr 28]. Available from: http://www.stat.fi/til/perh/index_en.html/
- HAEMACARE Working Group. Manual for coding and reporting haematological malignancies. Tumori. 2010;96:i-A32.
- Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat. 2006;15:651–674.
- Hothorn T, Zeileis A. Partykit: a modular toolkit for recursive partytioning in R. J Mach Learn Res. 2015;16:3905–3909.
- Breiman L, Friedman J, Olshen R, et al. Classification and regression trees. Monterey, CA: Wadsworth & Brooks/Cole Advanced Books & Software; 1984.
- Remes H, Martikainen P, Valkonen T. The effects of family type on child mortality. Eur J Public Health. 2011;21:688–693.
- Eurostat. Living conditions in Europe; 2014. [cited 2017 Aug 1]. Available from: http://ec.europa.eu/eurostat/documents/3217494/6303711/KS-DZ-14-001-EN-N.pdf/d867b24b-da98-427d-bca2-d8bc212ff7a8/
- Adam M, Rueegg CS, Schmidlin K, et al. Socioeconomic disparities in childhood cancer survival in Switzerland. Int J Cancer. 2016;138:2856–2866.
- Rudd RE. Health literacy skills of U.S. adults. Am J Health Behav. 2007;31(Suppl 1):S8–S18.
- van der Heide I, Wang J, Droomers M, et al. The relationship between health, education, and health literacy: results from the Dutch Adult Literacy and Life Skills Survey. J Health Commun. 2013;18 (Suppl 1):172–184.
- Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: a meta-analysis. Patient Educ Couns. 2016;99:1079–1086.
- de Looper M, Lafortune G. Measuring disparities in health status and in access and use of health care in OECD countries. Paris: OECD Publishing; 2009.
- OECD/EU. Health at a glance: Europe 2016: state of health in the EU Cycle. Paris: OECD Publishing; 2016.
