Abstract
Background: Hypofractionated (HRT) prostate radiation therapy has the potential to deliver a higher biologically effective dose over a shorter time compared with conventional fractionation (CRT). HRT, giving fewer fractions each with higher dose, might improve the therapeutic ratio, resource use and patient convenience but the toxicity is still controversial. Our objective was to compare the gastroinstestinal (GI) and genitourinary (GU) toxicity of HRT versus CRT.
Methods: Systematic review and meta-analysis of randomized clinical trials studies in PubMed, Cochrane and EMBASE databases published through December 2016 was done. Only randomized trials that evaluated patients with localized prostate cancer (PCa) undergoing CRT or HRT were included. In these studies, the daily dose was 1.8 Gy or 2 Gy per day for CRT and 2.4 to 3.4 Gy for HRT.
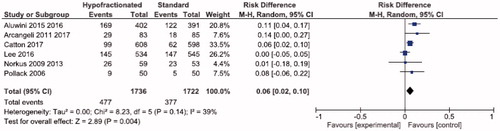
Results: 7317 patients in nine studies were analyzed. Six studies included acute GU toxicity data which showed similar rates for both HRT and CRT (32.6vs. 31.9%; RD 0.00; 95% CI; −0.03,0.03; p = .81; I2 = 0%). Similarly, seven studies showed no difference in late GU toxicity based on treatment schedule (28.7 vs. 28.0%; RD −0.01; 95% CI; −0.04,0.03; p = .67; I2 = 52%). GI toxicity at three months after radiotherapy was higher in patients treated with HRT in six studies (27.5 vs. 21.9%; RD 0.06; 95% CI; 0.02,0.10; p = .004; I2 = 39%); however, eight studies showed GI toxicity 12 months or more after radiotherapy that was statistically the same (12.9 HRT vs. 16.2% CRT; RD −0.01; 95% CI; −0.04,0.02; p = .41; I2 = 58%).
Conclusion: In meta-analysis of the available randomized trials on moderate HRT versus CRT for prostate cancer, acute and late GU toxicity were similar for both treatment schemes. While HRT was associated with higher acute GI toxicity, late toxicity was similar.
Introduction
Multiple studies, including randomized clinical trials, have shown that increasing radiation dose for prostate cancer improves local tumor control and decreases metastasis [Citation1]. Additionally, associated complication rates have been proven to be acceptable when advanced techniques such as intensity-modulated radiation therapy (IMRT) and image-guidance are used. This work was first done with conventional radiotherapy fractionation (CRT) and doses up to 79 Gy was used.
Based on evidence that prostate cancer has a low alpha/beta ratio, larger fraction size was thought to be a way to exploit the differential between tumor and normal tissue [Citation2]. Reports on the use of hypofractionated radiotherapy (HRT) schemes began to be published in the 1990s from countries with socialized medicine and in which treatment centers were sometimes distant from patients, such as the United Kingdom, Canada and Australia [Citation3]. Using moderately hypofractionated radiation schemes (less than 5 Gy/day) could also be beneficial in lowering treatment costs, decreasing the occupancy of linear accelerators leading to decreased waiting times for therapy and in the patient convenience of fewer fractions.
HRT for prostate cancer has the potential to deliver a higher biologically effective dose (BED) over a shorter time compared with CRT [Citation4]. However, the concern is the toxicity to the nearby rectum and bladder with the higher BED. Several randomized clinical trials reporting gastrointestinal (GI) and genitourinary (GU) toxicity have now been published. This is a systematic review and meta-analysis of the contemporary randomized clinical trials comparing moderate HRT to CRT focusing on toxicity outcomes.
Material and methods
Literature search
The search was performed using PubMed, Cochrane and Scielo for publications up to April 2017. Randomized trials published in English, Spanish or Portuguese were identified through a computerized blinded search using two sensitive search strategies combining the following medical subject headings and keywords: “((Radiotherapy or radiation therapy) and (prostate cancer OR prostatic neoplasm)). These were filtered by the “Titles/Abstract” category. All reviewed articles and cross-referenced studies from the retrieved articles were screened for pertinent information.
Inclusion and exclusion criteria
As seen in , we defined inclusion criteria for the literature search using the ‘Population, Intervention, Control, Outcome, Study Design (PICOS)’ approach.
Table 1. Studies characteristics.
Articles were selected for inclusion in the systematic review if they evaluated data from patients with localized prostate cancer treated with CRT or HRT. Randomized clinical trials with follow up of at least three months were included [Citation5–18]. Revisions and studies that did not report the outcomes of interest or adequate data were excluded [Citation1,Citation19–28].
When duplicate reports of the same study were found, data from the most complete report was included in the analysis. In studies of the same population but with different endpoints, we used the most recent article that offered the endpoint of interest [Citation5,Citation6,Citation10–16].
Data extraction
Data was independently extracted from each report by two authors (FKC and WB) according to the ‘Preferred Reporting Items for Systematic Reviews and Meta-analysis Statement (PRISMA)’ [Citation29], using a data recording form developed for this purpose. The data was reviewed and compared by a third author (AC). Instances of disagreement between the two extractors were resolved by reaching a consensus among the investigators. Whenever necessary, additional information about a specific study was obtained by directly questioning the main investigator.
Checklist and risk of bias
Two authors (FKC and WB) have applied the Scottish Intercollegiate Guidelines Network (SIGN) checklists for comparative studies and controlled trials for all articles included (Supplementary Appendix 1).
Definition of endpoints
The endpoints assessed included GU and GI toxicities as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events [Citation30]. The first three months were considered ‘acute events’, while events later than 12 months were considered ‘late events’. Only events greater than grade 1 were considered [Citation31].
Analysis
For all analyses, the longest follow-up for each trial was used. A pooled estimate of the risk difference in the individual studies was calculated using a random-effect model according to Mantel-Haenszel method and the results were graphically represented using forest plots. All of the results were presented with a 95% confidence interval (95% CI). The diamond at the bottom of the plot summarizes the best-estimate results of the meta-analysis, whose width represents the corresponding 95% CI.
The assumption of homogeneity was checked by a Chi-square test with the degrees of freedom (df) equal to the number of analyzed studies minus one. Additionally, the heterogeneity was measured by the I2, which describes the percentage of total variation across the studies that is due to heterogeneity rather than chance [Citation32,Citation33]. I2 was calculated from the basic results obtained from a typical meta-analysis as I2 = 100% × (Q − df)/Q, where Q is Cochran’s heterogeneity statistic. A value of 0% indicates no observed heterogeneity and larger values indicate increasing heterogeneity. If heterogeneity was detected (I2 > 50%), a possible explanation was sought. The presence of possible publication bias was investigated by a funnel plot and if a sub-analysis was possible it was performed to control this bias.
Evaluation of possible bias, as described by Daniel et al. [Citation34], was performed by two authors (FKC and WB). All analyses were conducted with Review Manager v5.0 (the Cochrane Collaboration’s Information Management System).
Results
Study selection
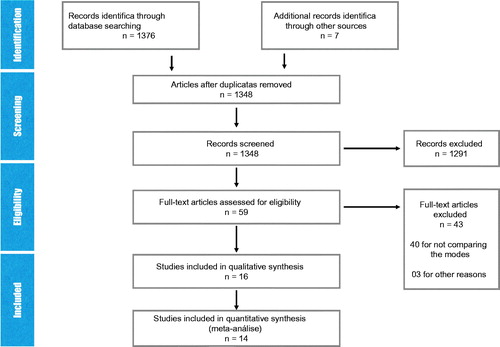
Our initial search identified 1376 articles and after the exclusion of 19 duplicate articles, 1357 studies were selected for detailed analysis of abstracts. 1298 studies were excluded since they did not fulfill the inclusion criteria. A detailed analysis of the remaining 61 articles was then performed. Forty were subsequently excluded due to inclusion criteria and three for incomplete data on the study population. Five articles were grouped as one because they used the same population ().
Study characteristics
Thirteen randomized trials were included for systematic review; nine studies containing 7317 patients ultimately remained in the analysis [Citation5–18].
Four studies were excluded because they did not supply adequate data [Citation19–22]. Lukka et al. [Citation19] and Yeoh et al. [Citation20] used the ‘National Cancer Institute of Canada’ and ‘Late Effect in Normal Tissue - Subjective Objective Management Analytic (LENT-SOMA)’ toxicities scale, respectively. These are not comparable with other included trials which used Radiation Therapy Oncology Group (RTOG) scale. While some of the nine selected articles contain the same study population, all were kept in the analysis in order to use the various information that each contributed. However, patient populations were not duplicated in any of the analyses (). The detailed analysis of bias from each study is present in Supplementary Appendix 1.
Synthesis of results
Acute and late GU toxicity
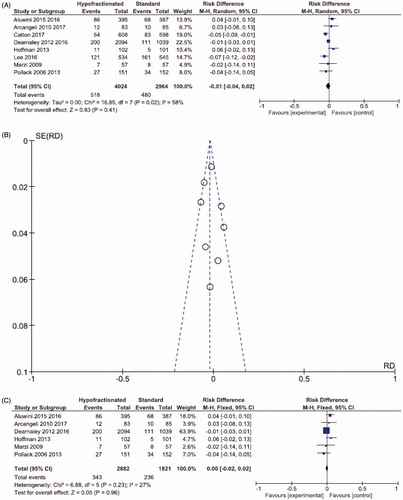
Three thousand four fifty nine patients from six studies were analyzed for acute GU toxicity. At three months after radiotherapy, acute GU toxicity was similar in patients treated with HRT and CRT (32.6 vs. 31.9%; RD 0.00, 95% CI; −0.03,0.03; p = .81; I2 = 0%; ).
Figure 2. Forest plot (A) Acute GU toxicity. (B) Late GU toxicity. (C) Funnel plot- late GU toxicity. (D) Forest plot- analysis of sensitivity: late GU toxicity.
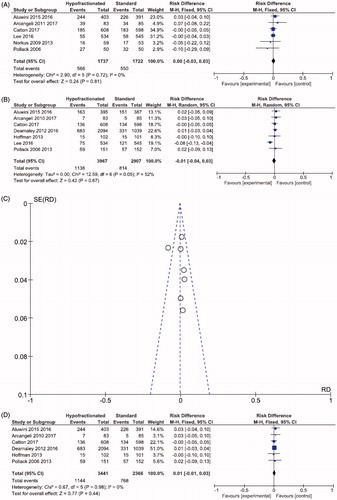
Seven studies and 6874 patients could be analyzed for late GU toxicity. At 12 months or more after radiotherapy, GU toxicity was also similar (28.7 vs. 28.0%; RD −0.01, 95% CI; −0.04,0.03; p = .67; I2 = 52%; ). Due to high heterogeneity, a sensitivity analysis was performed excluding one article [Citation17] and the six articles showed the same results (33.2 vs. 32.5%; RD −0.01; 95% CI; −0.01,0.02; p = .44; I2 = 0; ).
Acute and late GI toxicity
Using 3458 patients from six studies, GI toxicity at three months after radiotherapy was higher in patients treated with HRT versus CRT (27.5 vs. 21.9%; RD 0.06, 95% CI; 0.02,0.10; p = .004; I2 = 39%; ).
Nevertheless, eight studies with 6988 patients showed statistically similar GI toxicity at 12 months or more after radiotherapy in patients treated with HRT and CRT (12.9 vs. 16.2%; RD −0.01; 95% CI; −0.04,0.02; p = .41; I2 = 58%; ). Because of high heterogeneity, a sub analysis was performed. After excluding two articles [Citation13,Citation14], late GI toxicity remained similar between the schemes, HRT and CRT (11.9 = vs. 12.9%; RD 0.00; 95% CI; −0.02,0.02; p = .96; I2 = 27%; ).
Discussion
Emerging data from randomized trials evaluating post-radiotherapy toxicity in the treatment of localized prostate cancer showed mixed results when comparing HRT with standard schemes. The study reported here provides evidence that HRT has similar acute and late GU toxicity and late GI toxicity compared to conventional therapy, despite a higher rate of acute GI toxicity was seen. Only toxicity and not tumor control outcomes, was selectively analyzed because of the relatively short follow up interval available by which to definitively judge the latter. It is important though to make expeditious use of data which may reasonably drive clinical practice.
It has been hypothesized that prostate cancer has a low alpha/beta ratio which may be positively affected by higher dose per fraction with regard to local control without additional toxicity [Citation4]. Brenner and Hall [Citation35] analyzed two mature data sets, one using external-beam radiation therapy (EBRT) and other permanent radioisotopic implant, to determine the sensitivity of prostatic cancer to changes in fractionation using the linear quadratic model. The estimated alpha/beta ratio was 1.5 Gy for prostate tumor control. In a second publication, Brenner et al. [Citation4] estimated a similar alpha/beta ratio of 1.2 Gy and they concluded that appropriately designed hypofractionation schemes would be expected to maintain acceptable levels of tumor control and late sequelae, but with reduced acute morbidity, besides the logistic and financial advantage of fewer fractions. More recently, Vogelius et al. [Citation36] published a meta-analysis of 13 randomized trials with the aim of establishing the dose response and fractionation sensitivity of prostate cancer treated with different external beam radiation therapy fractionation schemes. Not taking the overall treatment time effect into account, they found an alpha/beta ratio of 1.2 Gy, as low as found by Brenner et al. [Citation4]. When the overall treatment time was taken into account, the alpha/beta ratio was a little higher (2.7 Gy).
Yeoh et al. [Citation20], in one of the first trials that compared HRT with CRT, showed similar results in late GU and GI toxicity at five years post treatment. In another pioneering trial, Lukka et al. [Citation19] confirmed the findings of similar late GU and GI toxicity in both treatment arms, HRT and CRT. Nevertheless, this study did show worse cancer control outcomes in the HRT group, although equivalent dose levels were low and preceded the dose escalation era.
Kuban et al. [Citation37] reported the preliminary outcome and toxicity of a phase III randomized clinical trial which showed no statistically significant difference in late GU and GI toxicity between HRT and CRT schedules. More recently, Arcangeli et al. [Citation12] reported the final results of a phase III randomized clinical trial which maintained the previously published three-year follow-up data, also with no significant difference in late toxicity between the two groups.
These findings are consistent with one meta-analysis and one systematic review [Citation24,Citation26] which were published in 2013 and 2015, respectively and both did not include the results from the most recently published randomized trials. In addition, the meta-analysis included studies [Citation19,Citation20] which used different toxicity scales such as the ‘(Late Effects of Normal Tissues (LENT)’ instead of the ‘National Cancer Institute Common Terminology Criteria for Adverse Events’, therefore it can increase heterogeneity of results. The same problem was seen with the recent meta-analysis by Cao et al. [Citation28] which reported similar acute GI toxicity with HRT and CRT, dissimilar to our analysis. However, this work contained higher heterogeneity (I2 > 50%) which was not controlled by sub-analysis as in our study. A more limited report by Franco et al. [Citation27] corroborated our results but included only three randomized clinical trials [Citation5,Citation6,Citation14], one of which was repeated [Citation5,Citation6]. Therefore, the meta-analysis reported here attempts to combine the most up to date, high level data on moderate hypofractionation schemes.
One factor which must be considered when comparing conventional versus hypofractionated schemes, both for tumor outcome and toxicity, is the BED. Of note is that some comparisons were of isoeffective regimens while others not only hypofractionated but also dose escalated. For example, the trial published by Lee et al. [Citation17] compared a lower equivalent dose in 2 Gy fractions (EQD2), 69.6 Gy, with a higher EQD2, 80 Gy, which of course can impact both tumor control and toxicity rates. Surely, this must be considered when interpreting clinical trial results. BEDs for the comparative study arms included in this meta-analysis are presented in .
Finally, this meta-analysis does have limitations. First, although all included studies utilized moderately HRT schemes, there was heterogeneity in dose per fraction, BED and treatment technique. Nevertheless, this is unavoidable due to the evolution of technology and the subsequent ability and perceived necessity to dose escalate. Second, the included population was different between studies with regard to initial risk stratification, although this would likely be more relevant for tumor control and survival outcomes which were not the focus of this analysis. Another limitation was our paper included only trials that described toxicity as measured by the Common Terminology Criteria for Adverse Events (CTCAE) (i.e., physician reported). Studies reporting patient reported outcome measures (PROM) were not included and based on this, there is a risk of underestimation of toxicity. The existing studies in the literature did not approach this question and further trials should extend its evaluation by PROMs. Finally, there was some heterogeneity in our meta-analysis, but it was appropriately controlled for through sensitivity analyses.
In conclusion, this systematic review and meta-analysis suggests that acute and late GU toxicity are similar for both CRT and HRT treatment schemes. While HRT was associated with higher acute GI toxicity, late toxicity was similar. Additionally, study results on tumor control have begun to show acceptable results for hypofractionated treatment when contemporary dose levels are used. This in conjunction with the added convenience and lower cost denotes that hypofractionated radiation for prostate cancer will become standard therapy.
Supplemental Material
Download PDF (171.8 KB)Acknowledgments
Ícaro T Carvalho and Willy Baccaglini had the same position as first authors in this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sun L, Zhu S, Zhao Y, et al. Who benefits from hypofractionated radiation therapy for clinically localized prostate cancer: evidence from meta-analysis. Tumour Biol. 2014;35:9911–9918.
- Katz A, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118.
- Hegemann NS, Guckenberger M, Belka C, et al. Hypofractionated radiotherapy for prostate cancer. Radiat Oncol. 2014;9:275.
- Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13.
- Pollack A, Hanson A, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526.
- Pollack A, Walker G, Horowitz EM, et al. Randomized trial of hypofractionated external-beam Rradiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–3868.
- Marzi S, Saracino B, Petrongari MG, et al. Modeling of α/β for late rectal toxicity from a randomized phase II study: conventional versus hypofractionated scheme for localized prostate cancer. J Exp Clin Cancer Res. 2009;28:117.
- Hoffman KE, Voong KR, Pugh TJ, et al. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: results from a randomized trial. Int J Radiat Oncol Biol Phys. 2013;88:1074–1084.
- Norkus D, Karkelyte A, Engels B, et al. A randomized hypofractionation dose escalation trial for high risk prostate cancer patients: interim analysis of acute toxicity and quality of life in 124 patients. Radiat Oncol. 2013;8:206.
- Arcangeli G, Saracino B, Gomellini S, et al. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol. 2010;78:11–18.
- Arcangeli G, Fowler J, Gomellini S, et al. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol. 2011;79:1013–1021.
- Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35:1891–1897.
- Dearnaley D, Syndikus I, Sumo G, et al. Conventional versus hypofractionated high-dose intensity modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13:43–54.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–6011.
- Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2015;16:274–283.
- Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. 2016;17:464–474.
- Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–2332.
- Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890.
- Lukka H, Hayter C, Julian J, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23:6132–6138.
- Yeoh E, Botten RJ, Butters J, et al. Hypofrartionated versus conventionally fractionated therapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–1278.
- Wilkins A, Mossop H, Syndikus I, etet al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16:1605–1616.
- Viani GA, Stefano EJ, Afosno SL, et al. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405.
- Strigari L, Arcangeli G, Arcangeli S, et al. Mathematical model for evaluating incidence of acute rectal toxicity during conventional or hypofractionated radiotherapy courses for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;5:1454–1460.
- Botrel TE, Clark O, Pompeo AC, et al. Hypofractionated external-beam radiation therapy (HEBRT) versus conventional external-beam radiation (CEBRT) in patients with localized prostate cancer: a systematic review and meta-analysis. Core Evid. 2013;8:1–13.
- Zaorsky NG, Ohri N, Showalter TN, et al. Systematic review of hypofractionated radiation therapy for prostate cancer. Cancer Treat Rev. 2013;39:728–736.
- Koontz BF, Bossi A, Cozzarini C, et al. A systematic review of hypofractionation for primary management of prostate cancer. Eur Urol. 2015;68:683.
- Franco RD, Borzillo V, Ravo V, et al. Rectal/urinary toxicity after hypofractionated vs. conventional radiotherapy in low/intermediate risk localized prostate cancer: systematic review and meta-analysis. Onco Target. 2017;8:17383–17395.
- Cao L, Yang YJ, Li ZW, et al. Moderate hypofractionated radiotherapy is more effective and safe for localized prostate cancer patients: a meta-analysis. Onco Target. 2017;8:2647–2658.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
- CTEP. USA. c2006. Available at https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctca.
- RTOG. Philadelphia. 2018. Available at https://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiation MorbidityScoringSchema.aspx.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
- Daniel RM, Cousens SN, De Stavola BL, et al. Methods for dealing with time-dependent confounding. Stat Med. 2013;32:1584–1618.
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101.
- Vogelius IR, Bentzen SM. Dose response and fractionation sensitivity of prostate cancer after external beam radiotherapy: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2018;100:858–865.
- Kuban DA, Nogueras-Gonzalez GM, Hamblin L, et al. Preliminary report of a randomized dose escalation trial for prostate cancer using hypofractionation. Int J Radiat Oncol Biol Phys. 2010;78:S58–S59.