Abstract
Background: Many patients experience toxicity from chemotherapy that may negatively impact their health-related quality of life (HRQoL), but side effects often go undetected by health care personnel. Our aim was to investigate whether hematologic toxicity (HT) was associated with HRQoL impairment, and, consequently, if blood counts could be used to identify patients with the highest need for supportive care during chemotherapy.
Material and methods: Data from two phase III trials of first-line chemotherapy in advanced non-small-cell lung cancer (NSCLC) were analyzed (n = 873). Blood counts were measured weekly in the treatment period. We categorized patients as having severe (CTCAE grade 3–4) or non-severe (grade 0–2) HT during the first chemotherapy cycle. HRQoL was reported on the EORTC QLQ-C30 and LC13 before and at the end of the cycle. The primary endpoints were changes in global quality of life, fatigue, nausea/vomiting and dyspnea (LC13).
Results: Of the 766 patients with complete data set, 177 (23%) developed severe HT during the first chemotherapy cycle. Changes in fatigue and nausea/vomiting were significantly worse for patients experiencing severe compared to patients with non-severe HT (difference in mean change of 4.9 points; p = .01, and 6.4 points; p = .01, respectively), but this association was limited to neutropenia, not thrombocytopenia or anemia. There were no significant associations between HT and global quality of life or dyspnea (difference in mean change of 2.1 points; p = .28, and 3.3 points; p = .053, respectively).
Conclusions: Patients developing severe HT had worse changes in two out of four of the primary HRQoL endpoints, but the association was not strong enough to use blood counts to identify patients who need more clinical attention and supportive care during chemotherapy.
Introduction
Cytotoxic chemotherapy remains an important treatment for advanced non-small-cell lung cancer (NSCLC), prolonging survival and improving and delaying cancer-related symptoms [Citation1, Citation2]. However, many patients experience significant toxicity that may negatively impact their health-related quality of life (HRQoL) [Citation3, Citation4]. Unfortunately, there are no good methods for predicting chemotherapy-induced impairment of HRQoL, and although adverse effects are common, up to half of treatment-related symptoms remain undetected by clinicians [Citation5, Citation6].
Hematologic toxicity (HT) is the main dose-limiting toxicity of chemotherapy, and causes some of the most important complications, such as neutropenic infections and thrombocytopenic bleedings. It seems reasonable that there might be an association between HT and HRQoL impairment, but few have investigated this and the results of previous studies are not consistent. Some have found reduced HRQoL in patients with chemotherapy-induced neutropenia, even in the absence of fever or other signs of infection [Citation7, Citation8], while others have not found associations between leukopenia/neutropenia and HRQoL [Citation9].
Blood counts are often controlled between courses of chemotherapy. If there are associations between HT and treatment-related symptoms, these laboratory tests could represent a simple and objective method for identifying patients at risk of severely impaired HRQoL who may benefit from supportive care during the treatment period.
In the present study, we reviewed data from two phase III trials of chemotherapy in advanced NSCLC [Citation10, Citation11]. The main aim was to investigate whether patients who experienced severe HT in their first treatment cycle had more negative changes in HRQoL than those who did not. Furthermore, the association between HT and survival was examined.
Methods
Patients and treatments
Both randomized clinical trials (RCTs) compared first-line chemotherapy regimens in advanced NSCLC [Citation10, Citation11]. All participants were chemotherapy naïve, had stage IV or IIIB not eligible for curative treatment, WHO performance status (PS) 0–2, and adequate bone marrow, kidney and liver function. Both trials were approved by ethics committees, and all patients gave written informed consent.
RCT 1 (n = 436) compared pemetrexed 500 mg/m2 plus carboplatin AUC =5 (Calvert’s formula) on day 1 (PC) with gemcitabine 1.000 mg/m2 on days 1 and 8 plus carboplatin AUC =5 on day 1 (GC) [Citation11]. RCT 2 (n = 437) compared vinorelbine capsules 60 mg/m2 plus gemcitabine 1.000 mg/m2 on day 1 and 8 (VG) with vinorelbine capsules 60 mg/m2 on day 1 and 8 plus carboplatin AUC = 5 on day 1 (VC) [Citation10]. While pemetrexed/carboplatin (PC) was administrated at day 1 only, the other treatments were administrated on days 1 and 8. Chemotherapy cycles were repeated every 3 weeks for up to four cycles in RCT 1 and three cycles in RCT 2. Patients who were 75 years or older had a 25% dose reduction from the first cycle.
Blood counts were performed weekly throughout the treatment period. HRQoL was reported on the European Organization for Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30) and the lung cancer-specific module LC13 [Citation12, Citation13]. The baseline questionnaire was completed before randomization. The next questionnaire was sent to the patient from the study office and completed immediately before the second cycle of chemotherapy (week 3). The recall period on the questionnaires was the last week.
Both RCTs reported similar survival and HRQoL outcomes, but differences in the frequency of HT. In RCT 1, patients receiving gemcitabine/carboplatin had more grade 3 or 4 neutropenia (PC: 40%, GC: 51%; p=.024) and thrombocytopenia (PC: 24%, GC: 56%; p<.001) [Citation11]. In RCT 2, patients receiving vinorelbine/carboplatin had more grade 3 or 4 neutropenia (VG: 23%, VC: 35%; p<.001) [Citation10].
Patient selection and design of present study
Patients were eligible for the present analyses if they received chemotherapy, had at least one blood count registered during the first cycle, and completed the HRQoL assessments at baseline and end of first cycle (week 3). Neutropenia, thrombocytopenia and anemia were graded using the Common Terminology Criteria of Adverse Events version 4.0. Severe HT was defined as grade 3 or 4 neutropenia (absolute neutrophil count <1.0 × 109 cells/L), thrombocytopenia (platelets <50 × 109/L) or anemia (hemoglobin <8.0 g/dL) occurring on any day during the first cycle.
The primary endpoints were changes in the scales pre-specified as the primary HRQoL endpoints in both RCTs [Citation10, Citation11]: global quality of life, fatigue, nausea/vomiting and dyspnea (LC13). Global quality of life gives information on the patient’s overall health status, fatigue and nausea/vomiting are common side effects of chemotherapy [Citation14], and dyspnea and fatigue are frequent symptoms in lung cancer [Citation3]. Changes in other HRQoL scales were reported as exploratory analyses.
Statistical considerations
The HRQoL scores were calculated according to the EORTC manual [Citation15]. A high score in global quality of life and on the functional scales represents a good health status, while a high symptom scale score represents more symptoms. To analyze the impact of HT on changes in HRQoL, we used linear mixed models for repeated measurements. The models included assessment time (baseline or end of cycle 1), HT (grade 0–2 or 3–4), the interaction term and the baseline score as fixed effects. Random intercepts for patients accounted for the dependence of repeated measurements. In addition, the effect of adjustment for chemotherapy regimen was investigated as this was considered to be the most relevant potential confounding factor. Since the main analyses included only patients who completed both the baseline and the follow-up HRQoL assessment, sensitivity analyses were performed including also patients with missing data at one of the assessments.
Previous studies have aimed to determine which changes in HRQoL scores that could be considered as clinically meaningful. In a study based on patients with breast and small-cell lung cancer, it was proposed that a mean change of 5–10 points corresponds to ‘a little difference’, 10–20 points to a ‘moderate difference’ and a change of more than 20 points to ‘a large difference’ [Citation16]. Other studies including patients with NSCLC have generally been in line with this proposal [Citation17]. Survival was defined as the time from randomization until death, and the groups were compared with the Kaplan–Meier method and the log-rank test. Median follow-up was estimated using the reverse Kaplan–Meier method. The level of statistical significance was defined as p<.05, and since this was an exploratory investigation, nominal levels of significance were reported without adjustments for multiple testing. Analyses were performed using Stata version 13.1 (College Station, TX, USA).
Results
Study population and hematologic toxicity
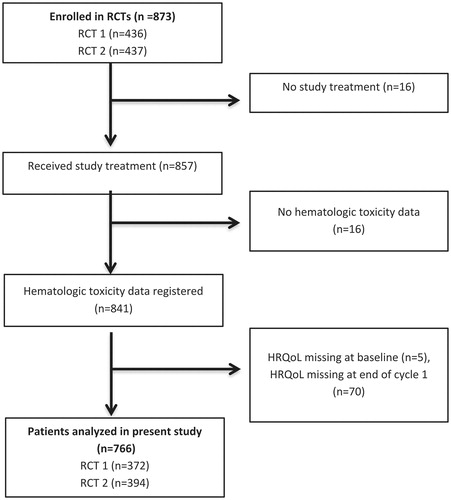
A total of 873 patients were enrolled in the two RCTs. Of the 841 patients that received study treatment and had at least one blood count in the first cycle, 766 (91%) patients completed both the baseline and the end of cycle 1 HRQoL assessment, and, thus, were eligible for the present study (). Reasons for exclusion were no study treatment (n = 16), missing data on HT during cycle 1 (n = 16) or missing HRQoL data at baseline (n = 5) or at the end of cycle 1 (n = 70).
Any severe HT was observed in 177 (23%) patients during the first cycle. Severe neutropenia and thrombocytopenia was observed in 149 (19%) and 67 (9%) patients, respectively. Only three (0.4%) patients had severe anemia. Of the 177 patients experiencing severe HT, 30 (17%) had severe HT on day 8, 165 (93%) on day 15 and six (3%) patients on day 21–22. Twenty-four (14%) patients experienced severe HT during more than one week of the cycle.
Among the patients with severe HT, there was a higher proportion of stage IIIB disease (28 vs. 20%; p=.03). There were no other statistically significant differences in baseline characteristics between those with severe HT and those without ().
Table 1. Patient characteristics at baseline according to severity of hematologic toxicity.
Relationship between hematologic toxicity and changes in HRQoL
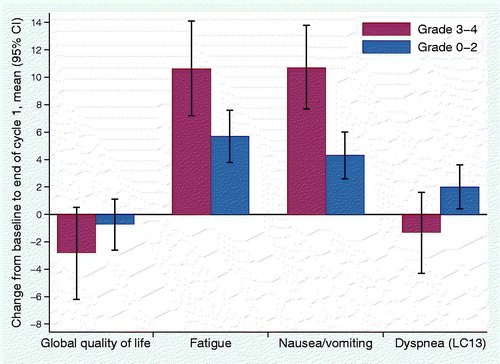
shows the mean change from baseline to end of cycle 1 in the primary endpoints according to worst grade of HT. Global quality of life decreased 2.8 points for patients with severe HT and 0.7 points for patients without (difference in mean change of 2.1 points; p=.28). Fatigue and nausea/vomiting mean scores increased more in patients with severe HT (10.6 vs. 5.7 points for fatigue; p=.01, and 10.7 vs. 4.3 points for nausea/vomiting; p=.01). For dyspnea (LC13), there was a trend towards improvement in patients with severe HT (1.3 points) and worsening in patients without (2.0 points), but the difference did not reach statistical significance (p=.053). Adjustment for chemotherapy regimen did not substantially influence these results, and the associations that were significant in the unadjusted analyses were significant also in adjusted analyses. Sensitivity analyses including all observed HRQoL data for the 841 patients with HT registrations gave similar results.
Figure 2. Change from baseline to end of cycle 1 in primary HRQoL endpoints for patients with and without severe hematologic toxicity.
For four of the remaining HRQoL scales, the analyses indicated that changes from baseline were significantly worse when patients experienced severe HT (). Role functioning, social functioning and alopecia worsened for all patients, but more for patients with severe HT (difference in mean change of 7.8 points for role functioning; p=.01, 4.5 points for social functioning; p = .046, and 4.8 points for alopecia; p = .02). Pain in arm or shoulder worsened in patients with HT and improved in those without (difference in mean change of 7.4 points; p = .01).
Table 2. Change from baseline to end of cycle 1 in HRQoL scales not defined as primary endpoints for patients with and without severe hematologic toxicity.
Analyses according to type of HT revealed that patients who experienced severe neutropenia had significantly worse changes in fatigue and nausea/vomiting, as well as role functioning, social functioning, cognitive functioning, alopecia, pain in arm or shoulder and pain in other parts compared to patients with no severe neutropenia (data not shown). Change in dyspnea (LC13) was more favorable for patients with severe neutropenia. Similar analyses for thrombocytopenia revealed no significant differences in the primary HRQoL endpoints, and for the remaining scales worse changes in role functioning only (data not shown). Since only three patients had severe anemia, we did not perform separate analyses in this group.
Overall survival
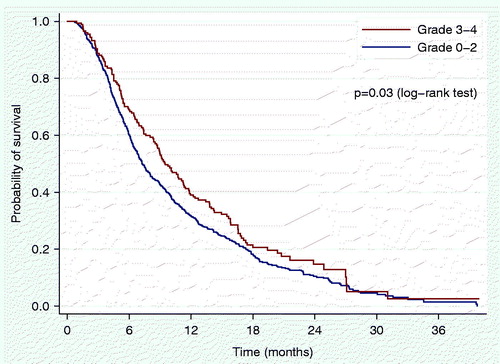
shows overall survival according to severity of HT. The median follow-up time for the survival analyses was 23.3 months, and 665 (87%) of 766 patients were registered as dead at database lock. The median survival was 9.5 months for patients with severe HT and 7.2 months for those without (p = .03).
Discussion
In this study, we found that patients with advanced NSCLC experiencing severe HT during their first cycle of chemotherapy had worse changes in fatigue and nausea/vomiting, but similar global quality of life and dyspnea as patients with no severe HT. Changes in role functioning, social functioning, alopecia and pain in arm or shoulder were also significantly worse for patients with severe HT. The differences in mean change were in the range of 5–10 points, corresponding to a clinically small difference [Citation16, Citation17].
There was a relationship between severe HT and impaired HRQoL for neutropenia, but not thrombocytopenia. An explanation for this may be that the underlying pathophysiological mechanisms for neutropenia and thrombocytopenia differ. Moreover, there are more complications of neutropenia than from thrombocytopenia. Unfortunately, we did not have data on the rate of objectively registered complications from neutropenia or thrombocytopenia after the first cycle of chemotherapy, since these complications were summarized for the whole study treatment period in both the RCTs from which we collected our data [Citation10, Citation11].
To our knowledge, no previous study has investigated whether there are associations between thrombocytopenia and HRQoL, but some have addressed the impact of chemotherapy-induced neutropenia. In two small studies (n = 80 and 84), Fortner et al. found that patients developing severe neutropenia had increased physical symptom distress and declined physical and social functioning [Citation7, Citation8]. In contrast, a large German multicenter study (n = 2.659) of docetaxel-related toxicities did not find any impact of leukopenia/neutropenia on global quality of life or other EORTC QLQ-C30 domains [Citation9]. An explanation for these divergent results may be the timing of the assessments relative to treatment administration and hematologic nadir. Fortner et al. assessed HRQoL weekly in the first treatment cycle, while in the German study HRQoL was assessed monthly, for up to 10 months. Acute side effects from chemotherapy are often transient, and we have previously demonstrated that the most severe HRQoL impairment is found in the first week following chemotherapy administration [Citation18]. In the present study, we did find some associations between HT and HRQoL impairment, but it is possible that the associations would have been stronger if HRQoL had been assessed at the time of the hematologic nadir.
Experiencing severe HT was also significantly associated with increased survival. This is in agreement with previous reports in many cancer types, focusing especially on chemotherapy-induced neutropenia [Citation19–21]. Drug disposition varies considerably between individuals, and it has been proposed that the absence of neutropenia (i.e., grade 0) indicates a weak biological effect of myelotoxic drugs [Citation19].
The results of the present study give some indications about the relationship between HT and HRQoL. Among the six HRQoL measures in which significant differences were detected, three (fatigue, nausea/vomiting and alopecia) are typical acute side effects of chemotherapy [Citation14]. This suggests that experiencing severe neutropenia is associated not only with increased survival, but also with more acute toxicity from chemotherapy. The social functioning and role functioning scales in the EORTC QLQ-C30 reflect whether the physical condition or treatment has interfered with family life and social activities, and the ability to do work, household or leisure activities. It stands to reason that this ability is lower in patients suffering more from chemotherapy toxicity. Pain in arm or shoulder is usually caused by the lung cancer rather than chemotherapy toxicity, and we have no good explanation why this was worse in patients with severe HT. It could be expected that neutropenia, as an indicator of biological effect, would be associated with improved control of cancer-related symptoms, but assessment at the end of the first cycle might have been too early to detect a symptomatic treatment response.
The results might have been different if we had analyzed subsequent cycles, but many patients have dose-reductions in later cycles and receive more supportive medication (e.g., antiemetics), and analyses and interpretation of the data are complicated by high dropout rates. These were the main reasons why we only analyzed the first cycle. By selecting patients that completed both HRQoL assessments, we performed a complete case analysis, which makes strong assumptions about the missing data mechanisms. However, we consider the proportion of patients with missing assessments as small (9%) and results of sensitivity analyses including all observed HRQoL data gave the same results and conclusions as the main analyses.
Conclusions
In conclusion, this study suggests that there may be greater decrements in certain HRQoL domains in patients experiencing severe neutropenia during chemotherapy. However, this association was not so strong that blood counts can be used to identify patients that need more clinical attention and supportive care during chemotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:3484–3515.
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v1–v27.
- Iyer S, Roughley A, Rider A, et al. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer. 2014;22:181–187.
- Sung MR, Patel MV, Djalalov S, et al. Evolution of symptom burden of advanced lung cancer over a decade. Clin Lung Cancer. 2017;18:274–280.e6.
- Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. JCO. 2004;22:3485–3490.
- Greimel ER, Bjelic-Radisic V, Pfisterer J, et al. Toxicity and quality of life outcomes in ovarian cancer patients participating in randomized controlled trials. Support Care Cancer. 2011;19:1421–1427.
- Fortner BV, Houts AC, Schwartzberg LS. A prospective investigation of chemotherapy-induced neutropenia and quality of life. J Support Oncol. 2006;4:472–478.
- Fortner BV, Schwartzberg L, Tauer K, et al. Impact of chemotherapy-induced neutropenia on quality of life: a prospective pilot investigation. Support Care Cancer. 2005;13:522–528.
- Al-Batran SE, Hozaeel W, Tauchert FK, et al. The impact of docetaxel-related toxicities on health-related quality of life in patients with metastatic cancer (QoliTax). Ann Oncol. 2015;26:1244–1248.
- Flotten O, Gronberg BH, Bremnes R, et al. Vinorelbine and gemcitabine vs vinorelbine and carboplatin as first-line treatment of advanced NSCLC. A phase III randomised controlled trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2012;107:442–447.
- Gronberg BH, Bremnes RM, Flotten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. JCO. 2009;27:3217–3224.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376.
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer (Oxford, England: 1990). 1994;30: 635–642.
- Feyer P, Kleeberg UR, Steingraber M, et al. Frequency of side effects in outpatient cancer care and their influence on patient satisfaction – a prospective survey using the PASQOC questionnaire. Support Care Cancer. 2008;16:567–575.
- Fayers PM, Aaronson N, Bjordal K. EORTC QLQ-C30 scoring manual. 3rd ed. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 2001.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. JCO. 1998;16:139–144.
- Maringwa JT, Quinten C, King M, et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer. 2011;19:1753–1760.
- Kristensen A, Solheim TS, Amundsen T, et al. Measurement of health-related quality of life during chemotherapy – the importance of timing. Acta Oncol (Stockholm, Sweden). 2017;56:737–745.
- Di Maio M, Gridelli C, Gallo C, et al. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 2005;6:669–677.
- Pallis AG, Agelaki S, Kakolyris S, et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with advanced non-small cell lung cancer treated with front-line docetaxel-gemcitabine chemotherapy. Lung Cancer (Amsterdam, Netherlands). 2008;62:356–363.
- Rambach L, Bertaut A, Vincent J, et al. Prognostic value of chemotherapy-induced hematological toxicity in metastatic colorectal cancer patients. WJG. 2014;20:1565–1573.