Abstract
Objective: Clinical stage (c-stage) at diagnosis is the most significant prognostic marker for patients with cancer, where 1- and 5-year survival rates as main landmarks when assessing outcomes. This is a population-based case study of Danish c-stage I lung cancer patients who were considered candidates for curative therapy and then died within 1 year after diagnosis (cases). Cases were identified in the Danish Lung Cancer Register (DLCR), and medical records were used to retrieve treatment details and cause of death (CoD). Our aims were, if possible, to identify and describe clusters of patients, in terms of CoD and treatment modality at risk for an adverse short-term outcome.
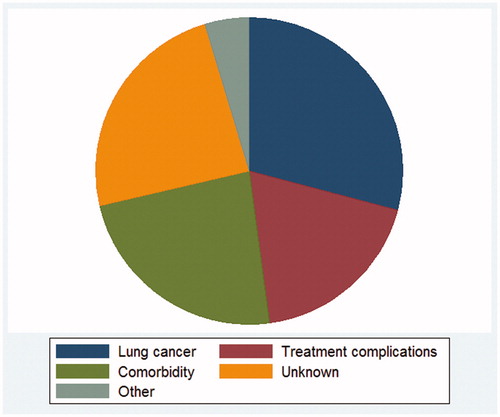
Results: Patients who died early were more frequently male, older, had squamous-cell histology, were less frequently surgically treated and generally had a higher burden of comorbidity. In terms of CoD, 29% died of lung cancer with distant recurrence (DR) as the most common type of recurrence (55%). Death from co-morbidity occurred for 23%, where the largest proportion (36%) died from another cancer. Nineteen percentage died from treatment complications, with the majority being male (p < .001). The remainder died of unknown or other causes.
Conclusions: Lung cancer with DR remains the most common CoD. Identifying and accordingly treating patients at risk for DR could potentially improve outcomes. Further studies of the predominantly male subgroup of patients who die of treatment complications are needed. Death from co-morbidity especially in patients with another cancer is a significant CoD and when assessing the quality of lung cancer care a competing event.
Background
Globally, lung cancer remains the most common cause of cancer related death [Citation1]. Compared to other Nordic countries, Denmark has a high lung cancer incidence and Danish lung cancer patients have historically had a high mortality [Citation2]. Currently, the most significant prognostic factor is stage based on tumor, lymph node status and metastasis (TNM) at diagnosis [Citation3]. To improve cancer survival, several national initiatives to diagnose lung cancer at an earlier stage have been implemented in Denmark [Citation4]. For stage I lung cancer, the treatment of choice is surgery [Citation5]. In addition to stage, several other factors affect the prognosis and available treatment options, such as tumor histology, age, gender, comorbidity, life style factors, socioeconomic factors and treatment complications [Citation6–9]. However, effective treatment alternatives to surgery exist for stage I lung cancer [Citation10,Citation11], thus providing treatment options with curative intent also for patients who are not candidates for surgery.
One-year survival and 5-year survival are usually considered the main landmarks when evaluating cancer outcomes [Citation12]. The aim of the present study was to establish the cause of death (CoD) in Danish clinical stage (c-stage) I lung cancer patients (TNM 7th edition) [Citation3] who were considered candidates for curative therapy but nevertheless died within 1 year after diagnosis and – if possible – to identify clusters of patients at risk for early death.
Methods
This is a population-based case study. The Danish Lung Cancer Registry (DLCR) has since 2000 collected data on diagnostic work-up, staging and treatment on lung cancer patients from all 29 departments involved in the diagnosis and treatment of lung cancer in Denmark; historically, it has had a completeness of 90% [Citation13]. Through linkage with other national registries, it also contains information on vital status and date of death. The DLCR is one of the two national registries for lung cancer, the other being the Danish cancer registry, which contains fewer clinical variables. Reporting to this register is mandatory, and the degree of completeness is high; however, the exact number has not been reported [Citation14].
From the DLCR, we identified c-stage IA or IB lung cancer patients diagnosed from 2011 through 2014. Among these, we identified cases as patients who, according to DLCR, were treated with curative intent, but died within 1 year after diagnosis. Since data on comorbidity were not available from the DLCR, we aimed to match the cases with two controls that survived more than 1 year, according to stage, age (5-year increments), gender, type of treatment and year of diagnosis (either index or previous).
We retrieved medical records from the departments of pulmonology, thoracic surgery and oncology in order to validate the register-based data, confirming date of diagnosis, c-stage and treatment intent. Furthermore, we obtained information about treatment, complications, comorbidity, recurrence and CoD. Final inclusion was done after the review of the medical records according to the following inclusion criteria: patients with c-stage IA or IB lung cancer who were found to be a candidate for treatment with curative intent, as concluded by the multidisciplinary team conference at the end of the diagnostic work-up or at referral to curative treatment. Curative treatment modalities were surgery, stereotactic body radiotherapy (SBRT) and conventional chemoradio therapy.
Based on the medical records, we classified CoD into ‘lung cancer’, ‘treatment complications’, ‘comorbidity’ (including other primary cancers) or ‘unknown’ and other ‘causes’ (accidents, suicide and causes that could not be categorized into the other specific causes). The CoDs according to our classification were then compared to those registered on the death certificate (DC).
Death was regarded as caused by lung cancer if the patient died of lung cancer during admission or if the patient was referred to palliative care including hospice or another hospital department due to disease progression at the last recorded follow-up visit. If the patient died perioperatively or developed either a long lasting or what was considered a delayed complication to treatment, ie, chronic infection, air leak or radiation pneumonitis, death was considered as caused by treatment complications. We applied no time limitation for experiencing a treatment complication.
Based on the medical records, we established the burden of comorbidity both for the cases and the matched controls using the Charlson Comorbidity Index (CCI) [Citation15].
Statistical analyses
Since data were reported to the DLCR at the same time point during the course of disease – before the cases had died, a case cohort approach was used. We conducted a comparison of baseline parameters: age, gender, clinical TNM stage, tumor histology and the fraction of patients having surgery, between the cases and the entire c-stage I DLCR population and furthermore in terms of comorbidity for the matched controls. This was done in order to describe to what extent the known risk factors for outcome apply to our cases.
Proportional distribution was calculated and compared where relevant. Age means were compared using the Student’s t-test, and CCI scores were compared by the Mann–Whitney U test. Calculations were performed with SAS Software (SAS system; SAS Institute, Cary, NC, USA) or Stata software (StataCorp 4905; College Station, TX, USA).
Results
According to DLCR, 2985 patients were diagnosed with c-stage I lung cancer from 1 January 2011 to 31 December 2014, of whom 382 (13%) died within the first 365 days after diagnosis. Two hundred sixty-nine of the 382 patients (70%) had a treatment registration in the DLCR. We were able to retrieve the MR from 253 patients. After review, we found that 19 patients either did not have lung cancer or had a more advanced stage. These patients were excluded from both the case group and the DLCR cohort (n = 2966). Another 13 cases had not been candidates for curative therapy and were excluded from the case group, which then finally included 221 patients. These patients were ultimately matched with 410 controls (262 surgically and 148 oncologically treated patients).
Of the 221 cases considered candidates for curative therapy at the MDT, eight patients (4%) actually received therapy with palliative intent; four patients were due to disease progression at start of planned treatment; three patients were due to comorbidity and one patient was due to tumor location. Thus, 213 cases initiated therapy with curative intent, 127 (60%) cases had surgery and 86 (40%) cases initiated oncological treatment.
Baseline parameters
The main findings are presented in . Patients who died early were significantly older compared to the entire DLCR cohort, and the male/female ratio was reversed. In the case group, there were 13% point more men than women (p = .006). The fraction in the case group having surgery was significantly lower as compared to the entire cohort: 57 vs 68% (p = .013). Regarding histology, a smaller proportion of cases had adenocarcinoma and a larger proportion had a squamous cell carcinoma.
Table 1. Descriptive characteristics at time of diagnosis according to treatment modality for the patients who died within 1 year and the full population of stage I lung cancer patients diagnosed in Denmark, 2011–2014 (DLCR cohort).
Regarding comorbidity, the average CCI among the case group was 2.3 vs 1.3 in the control group (p < .001) and the association remained in subgroup analyses according to either surgery (p = .001) or curative oncological treatment (p = .004). Moreover, the CCI among the surgically treated cases was significantly lower than the oncologically treated cases (p = .002).
The overall distribution of CoD for the 213 cases is shown in .
The surgically treated
Of the cases having surgery, 63% were males (95% CI 55–71%) as compared to 45% (95% CI 43–47%) males among those that had surgery in the DLCR cohort (n = 2021). Of the patients dying of treatment complications (n = 38), 76% were males (95% CI: 63–90%), which was significantly larger than the proportion of women (p < .001). Of cases having surgery and dying of comorbidity (n = 23), 11 were female and 12 were male.
As shown in , the main types of surgery were lobectomy and wedge resection. Among patients who had a lobectomy, the two most common causes of death were lung cancer and treatment complications with no significant difference between the number of deaths from the two causes (p = .30). The third most common cause of death was comorbidity.
Table 2. The cause of death by type of curative treatment among stage I lung cancer patients, diagnosed 2011–2014, who died within a year of diagnosis.
Among patients who had a wedge resection, lung cancer and comorbidity were the two most common causes of death, while treatment complications were the third. However, the numbers of death by the three causes were not significantly different in this group.
We compared the group specific proportions by CoD between the lobectomy and wedge group. Our most notable finding was that the proportion of patients dying from comorbidity was 18% point (95% CI 1–35%) higher in the wedge resection group as compared to the lobectomy group (p = .023). There was no significant difference in the proportions of patients dying of lung cancer and treatment complications between the lobectomy and wedge groups.
The oncologically treated
Comorbidity and lung cancer were the main causes of death among patients treated with stereotactic body radiotherapy (SBRT), with no significant difference between the numbers of deaths by the two causes (p = .48). The main difference in causes of death between patients receiving SBRT and patients receiving surgical treatment was the significantly lower occurrence of death due to treatment complications in the SBRT group compared to the wedge and lobectomy groups (p = .03 and p < .001, respectively).
Patients dying of lung cancer
Of the 62 (29%) cases dying of lung cancer (), 56 (26%) cases had completed curative treatment, were considered cancer free and yet died of lung cancer and thus had recurrence. Thirty-one (55%) cases had distant recurrence (DR), 15 (27%) cases had combined locoregional and DR, 7 (13%) cases had nodal recurrence, 2 (4%) cases had local recurrence and one (2%) case had recurrence at unknown site.
There was no statistical significant difference in the pattern of recurrence between cases treated with surgery or SBRT (data not shown).
Complications to cancer treatment
Forty (19%) of the 213 patients treated curatively died of treatment complications, and 95% of these patients were surgically treated. It is beyond the scope of this article to categorize the types of complications further.
Patients dying from comorbidity, unknown - and other causes
Fifty patients (23%) died of co-morbidity. The main cause in this group was death from another primary malignant disease (n = 18, 36%). Of these cancers, 10 (56%) originated from the gastro-intestinal tract, while renal cancer and melanoma were also observed. The majority, (n = 14, 78%) of the patients dying of another cancer had been diagnosed with this prior to the diagnosis of the lung cancer. Eight (16%) patients died from cardiac disease, 7 (14%) patients died from nonmalignant gastrointestinal comorbidity, 5 (10%) patients died from pulmonary comorbidity, 4 (8%) patients died from neurologic comorbidity, 4 patients died from sepsis, 2 (4%) patients died from ruptured aortic aneurisms and 2 patients died from peripheral vascular disease. Among the 10 patients dying of ‘other’ causes, one patient committed suicide, another patient died of an accidental intravenous drug overdose during in hospital treatment and one patient died of complications to the diagnostic work-up.
In the rest of the cases, the cause of death could not be categorized into any of the other categories, but 50% of the patients in this category had progression of their lung cancer prior to death.
Cause of death, the death certificate vs medical record
CoD was lacking in 35 (16%) of the DCs, and the CoD could not be established from the MR of 53 (24%) of the 221 early death patients. Thus, among 134 patients dying from either lung cancer (including treatment complications) or comorbidity, on whom we had complete data from both the DC and the MR, the correlation between the two data sources was 82%. When using MR as reference, the sensitivity of identifying a lung cancer CoD from the DC was 85%. The specificity of no lung cancer registered on the DC was 73%. Our findings correspond to a positive predictive value (PPV) of a lung cancer-related death on the DC of 88% and a negative predictive value (NPV) of no lung cancer on the DC of 68%. Among patients dying of treatment complications (n = 40), 37 had a CoD registered on the DC. Of them, 10 (27%) patients did not have a lung cancer ICD-10 code on the DC. Among the 53 patients with unknown CoD in the MR, 27 (50%) patients died of lung cancer-related causes and 14 (26%) patients died of comorbidity according to the DC.
Discussion
In a population-based setting, we have characterized a stage-specific group of patients, who were candidates for curative therapy but nevertheless died early after lung cancer diagnosis. In contrary to other studies, we have not limited us to only one treatment modality.
The most common cause of early death in our study was lung cancer. This is in accordance with a recent Norwegian single-center study of 756 surgically treated patients [Citation16] and an older American study of 5371 surgically treated patients [Citation17]. More than half of the lung cancer deaths in our study had DR. It could be argued that these patients had micro-metastases at treatment onset. Local recurrence as an indicator of treatment failure occurred only in a minority of patients (4%). This corroborates that the treatment modalities are efficient at achieving initial local disease control. Efforts should be made for detecting micro-metastases at the time of diagnosis [Citation18,Citation19] even in c-stage I patients, as these should rather have been given systemic oncological treatment or efforts should have been undertaken to eliminate the metastasis as part of the curative effort. In terms of pretreatment staging, the concordance between the clinical and pathological TNM was between 90.5 and 92.6 during the study period [Citation20], which is significantly higher than the 59.6% reported by a Dutch prospective study of 1779 c-stage I patients diagnosed in 2013 and 2014 [21].
Almost one-fifth of patients died of treatment complications (19%), which thus remain a significant cause of early death, especially for the surgically treated men. Studies have pointed to improved surgical technique, operator training and centralized treatment in high volume centers to improve outcome and reduce treatment complications [Citation22]. However, the surgical treatment of lung cancer in Denmark is highly centralized into four high volume centers performing from 127 to 274 lung cancer operations annually (2014) [20] and the primary surgical technique is video assisted thoracic surgery. To which extent improvements, in terms of organization, can be achieved is questionable, since the 30-day survival for operated patients in the study period was 98-99% [Citation20]. A notable finding was that lung cancer-related ICD-10 codes were missing in 27% of the patients who died of treatment complications, which correspondingly results in a low negative predictive value of a noncancer-related CoD in the DC.
Death from comorbidity was the third most common cause of early death. More than every four patients in this group died of a preexisting cancer (28%) of which the majority originated from the GI tract. We observed that many of the patients with a preexisting cancer were diagnosed with lung cancer via the follow-up or diagnostic work-up for the other cancer. Consequently, they would tend to have a poor prognosis, even though they are diagnosed with an early-stage lung cancer. The treatment of patients with multiple cancers is complex, and to decide on the optimal choice of treatment and sequence of interventions is challenging. It can be argued that in the patients we identified, the outcome was dictated by the poor prognosis of the preexisting cancer. So whether it is appropriate and beneficial to offer curative treatment to stage I lung cancer patients with another primary cancer should be carefully considered.
Noncancer comorbidity probably contributes to patients dying of treatment complications [Citation23]. Comorbidity also affects the choice of treatment, especially for stage I patients, and is thus a confounder by indication, as indicated by the observed difference in CCI between the surgically and oncologically treated cases in our study.
As long as, lung cancer recurrence and treatment complications remain the most common causes of early death in Danish stage I lung cancer patients, there is room for an improvement in lung cancer care and treatment selection. Further studies into patient-related factors that affect the prognosis, while taking into account the known risk factors (age, gender and treatment), are needed.
Strengths and limitations
The major strength of this study is that it is population-based. Furthermore, we have used medical records as the primary data source to improve the quality and level of detail of the data, especially regarding the post-treatment phase, for which DLCR and other available registers lacks information. Furthermore, by combining register-based data with patient-level data, we have reduced the risk of misclassification bias of the cases.
Our study still has limitations. In contrary to the case/noncase study, the case-cohort approach is prone to underestimate differences between the two groups since the cases are also included in the entire DLCR cohort. However, given there mere size of the DLCR cohort, we believe that this is of less importance in the present study.
Based on the M,R we were unable to establish the CoD in a fourth of the patients. However, according to the DC, the causes in these cases were primarily due to lung cancer or comorbidity. Consequently, their respective fractions in CoD are probably higher than what we have reported. Information on comorbidity was based on data from hospital records, and we did not have access to data from the general practitioner, which may have caused us to underestimate the CCI. However, we believe this potential misclassification to be nondifferential.
Misclassification of a treated patient as untreated (no treatment registration) in the DLCR would consequently mean that eligible patients could have been missed. Thus, our findings are limited to those with a treatment registration in the DLCR. The identification of the study population was done solely on the DLCR. We could potentially have identified additional potential study subjects if we had included the Danish Cancer Registry [Citation14]. We are not able to assess potential under-reporting to the DLCR. However, since we have included only treated patients, hence, any study subject had contact with at least two departments who report data to the DLCR, we find it unlikely that bias from under-reporting has significantly affected the results of the present study.
Conclusion
The primary cause of early death in Danish stage I lung cancer patients who are treated curatively remains lung cancer, while primary competing events are death caused by treatment complications and co-morbidity. Further studies into identifying patients at risk for early relapse after successful local treatment are warranted. A further characterization of the predominantly male subgroup of patients dying of treatment complications is also needed and the results could potentially improve treatment selection for the individual patient. Assessing the importance of comorbidity in relation to lung cancer is complex since it affects both treatment selection and outcome. Our preliminary findings suggest that local control of stage I lung cancer may be of limited value in patients with a gastrointestinal cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kay FU, Kandathil A, Batra K, et al. Revisions to the tumor, node, metastasis staging of lung cancer (8th edition): rationale, radiologic findings and clinical implications. World J Radiol. 2017;9:269. doi:10.4329/wjr.v9.i6.269.
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax. 2013;68:551–564. doi:10.1136/thoraxjnl-2012-202297.
- Mirsadraee S. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4:0. doi:10.4329/wjr.v4.i4.128.
- Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians – a national Danish project. Health Policy. 2012;105:65–70. doi:10.1016/j.healthpol.2011.11.001.
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:7S–37S. doi:10.1378/chest.12-2377.
- Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi:10.1016/S0140-6736(13)61502-0.
- Caposole MZ, Miller K, Kim J, et al. Elimination of socioeconomic and racial disparities related to lung cancer: closing the gap at a high volume community cancer center. Surg Oncol. 2014;23:46–52. doi:10.1016/j.suronc.2014.02.001.
- Adham M, Kurniawan AN, Muhtadi AI, et al. Morbidity, mortality, and categorization of the risk of perioperative complications in lung cancer patients. J. Bras. Pneumol. 2014;40:185–196. doi:10.2337/db06-0618.
- Deleuran T, Thomsen RW, Nørgaard M, et al. Comorbidity and survival of Danish lung cancer patients from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5:31–38.
- Mehta HJ, Ross C, Silvestri G, et al. Evaluation and treatment of high-risk patients with early-stage lung cancer. Clin Chest Med. 2011;32:783–797. doi:10.1016/j.ccm.2011.08.011.
- Heuvelmans MA, Groen HJM, Oudkerk M. Early lung cancer detection by low-dose CT screening: therapeutic implications. Expert Rev Respir Med. 2017;11:89–100. doi:10.1080/17476348.2017.1276445.
- McPhail S, Elliss-Brookes L, Shelton J, et al. Emergency presentation of cancer and short-term mortality. Br J Cancer. 2013;109:2027–2034. doi:10.1038/bjc.2013.569.
- Jakobsen E, Green A, Oesterlind K, et al. Nationwide quality improvement in lung cancer care: the role of the Danish Lung Cancer Group and Registry. J Thorac Oncol. 2013;8:1238–1247.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45. doi:10.1177/1403494810393562.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8.
- Bugge AS, Lund MB, Valberg M, et al. Cause-specific death after surgical resection for early-stage non-small-cell lung cancer. Eur J Cardiothorac Surg. 2018;53:221–227. doi:10.1093/ejcts/ezx274.
- Eguchi T, Bains S, Lee M-C, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage I non-small-cell lung cancer: a competing risks analysis. J Clin Oncol. 2017;35:281–290. doi:10.1200/JCO.2016.69.0834.
- Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J Thorac Oncol. 2011;6:43–47. doi:10.1097/JTO.0b013e3181f9abca.
- Iskender I, Kadioglu SZ, Cosgun T, et al. False-positivity of mediastinal lymph nodes has negative effect on survival in potentially resectable non-small cell lung cancer. Eur J Cardio Thoracic Surg. 2012;41:874–879. doi:10.1093/ejcts/ezr054.
- Dansk Lunge Cancer Register. Indikatorrapport til National årsrapport 2014;2.
- Heineman DJ, Geert M, Daniels JM, et al. Clinical staging of stage I non-small cell lung cancer in the Netherlands – need for improvement in an era with expanding nonsurgical treatment options: data from the Dutch Lung Surgery Audit. Ann Thorac Surg. 2016;102:1615–1621. doi:10.1016/j.athoracsur.2016.07.054.
- Lüchtenborg M, Riaz SP, Coupland VH, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol. 2013;31:3141–3146. doi:10.1200/JCO2013.49.0219.
- Green A, Hauge J, Iachina M, et al. The mortality after surgery in primary lung cancer: results from the Danish Lung Cancer Registry. Eur J Cardio-Thoracic Surg. 2016;49(2):589–594. doi:10.1093/ejcts/ezv107.