Abstract
Objectives: First-line chemotherapy for metastatic colorectal cancer (mCRC) is effective and feasible in selected older patients. We investigated age-dependent differences in treatment and outcomes in patients with mCRC in clinical practice.
Material and methods: A retrospective study of 654 patients with mCRC referred to first-line chemotherapy in 2008–2014. Patients were divided into two age groups: 50–69 and ≥70 (older patients). Binary outcomes were analyzed by logistic regression. Progression-free survival (PFS) and overall survival (OS) were analyzed by Cox proportional hazards regression, CRC-specific and other-cause mortality with Fine and Gray proportional hazard model for the sub-distribution of a competing risk.
Results: After adjusting for performance status (PS) and comorbidity, older patients were more likely to receive monotherapy (adjusted odds ratio (aOR) = 9.00, 95% confidence interval (CI) 4.52–17.91), lower doses, and no additional targeted therapy (aOR = 1.89, 95% CI 1.28–2.78) than younger patients. Yet, older patients experienced more toxicity and hospitalizations (aOR = 1.53, 95% CI 1.08–2.17). Among those treated, older patients had shorter PFS (hazard ratio (HR) = 1.32, 95% CI 1.11–1.57), but after adjusting for PS and comorbidity, PFS was similar. No significant difference was found in CRC mortality (HR = 1.15, 95% CI 0.95–1.40) between age groups. Poor PS was associated with shorter OS and PFS and higher CRC mortality.
Conclusions: In the DISCO study, older patients with mCRC received less aggressive first-line chemotherapy. Yet, they experienced more toxicity. Younger and older patients had similar CRC mortality. Shorter PFS and higher CRC mortality were observed in patients with poor PS.
Introduction
The incidence of colorectal cancer (CRC) increases throughout life [Citation1], and as populations are aging [Citation2], the number of older patients with metastatic CRC (mCRC) grows rapidly. New surgical techniques, combination chemotherapy, and targeted therapy have improved survival in patients with CRC, albeit most markedly in younger patients. In clinical trials, overall survival (OS) has increased from 6 to 8 months to 24 months for patients with mCRC treated with combinations of chemotherapy and biological drugs [Citation3,Citation4]. However, in everyday clinical practice, the median survival is only 10–11 months [Citation5].
Many patients with mCRC do not receive chemotherapy because of high age, comorbidity, poor performance status (PS), and concerns regarding toxicity and efficacy [Citation6]. Nevertheless, chemotherapy seems effective and feasible in selected older patients, and clinical trials have shown that irinotecan/5-fluorouracil (5-FU) combinations are as well tolerated and effective in both older and younger patients [Citation7,Citation8]. In daily clinical practice, combination chemotherapy is associated with prolonged progression-free survival (PFS) in older patients, although ∼70% of all patients require dose reductions [Citation9]. Conversely, a randomized trial reported only non-significant improvements in PFS and OS in frail older patients when irinotecan was added to 5-FU, but with an increased risk of grade 3–4 toxicity [Citation10], which has also been found when reduced doses of oxaliplatin were added to capecitabine or 5-FU [Citation11]. The addition of bevacizumab to chemotherapy in older patients improves PFS [Citation12] and OS [Citation13], but increases thromboembolic risk [Citation13,Citation14]. Furthermore, the efficacy and safety of cetuximab and panitumumab seem to be independent of age [Citation15], but evidence regarding anti-EGFR therapy in older patients is generally missing.
Older patients have a higher risk of toxicity [Citation16,Citation17], but age should not be an exclusion criterion for chemotherapy [Citation18,Citation19]. Comorbidity and PS, but not age alone, influence treatment outcomes in the adjuvant setting [Citation20], and decreased PS is associated with poorer outcomes in the palliative setting [Citation21]. Oncologists and geriatricians have begun to cooperate to improve treatment in older patients with cancer. Comprehensive geriatric assessment (CGA), the gold standard for assessing the physical and psychological health, functional status and socioenvironmental parameters of an older adult, is recommended before deciding on monotherapy or combination therapy in patients with mCRC [Citation22]. However, CGA is far from being widely implemented in oncological clinical practice. To our knowledge, there are no studies that consequentially have evaluated true age-dependent differences and only a few studies have evaluated the association between reduced doses of chemotherapy and treatment outcomes in older patients with mCRC [Citation23].
The DISCO study investigates age-dependent differences in treatment and outcomes in patients referred to first-line chemotherapy for mCRC, with the hypothesis that older patients with mCRC receive less aggressive treatment, experience more toxicity, and have a worse prognosis than younger patients.
The DISCO study is to our knowledge the first study to evaluate true age-dependent differences by consequently adjusting for PS and comorbidity, to distinguish age-dependent differences from differences due to PS and comorbidity, which often is associated with age.
Material and methods
The DISCO study is a retrospective single-center study evaluating age-dependent differences in treatment and outcomes of first-line chemotherapy in patients with mCRC treated in 2008–2014 at the Department of Oncology, Herlev Hospital, Denmark.
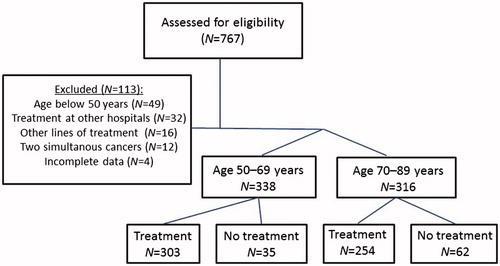
Patients were screened for eligibility by reviewing the medical charts of all patients with mCRC referred to first-line chemotherapy during the period. In total, 113 patients were excluded for various reasons, and 654 patients were eligible for inclusion in the present study (). Patients <50 years were excluded from the study, because mCRC in younger patients seems to differ from that in older patients: CRC in young patients appears to be more aggressive, to be at a more advanced stage at presentation, and to have poorer pathologic findings [Citation24]. Furthermore, age has been found to be prognostic for OS and PFS, with poorer prognosis for both younger and older patients, the best prognosis being for 57-year-old patients [Citation21].
Data were extracted and interpreted by one physician. Baseline characteristics included demographic data, comorbidities, body mass index (BMI), civil status, PS, and tumor characteristics.
The cause of death was categorized into CRC mortality, treatment-related mortality, and other causes of death by reviewing medical records. Electronical medical records from all patients were identified by the unique 10-digit personal identification number (PIN) assigned to all Danish citizens at birth or upon immigration, which permitted follow-up of all patients, even if admitted to other hospitals. We defined CRC-related mortality as all deaths in patients described as ‘terminal’. If a patient’s death was due to something other than CRC (toxicity, other medical conditions, or unknown cause of death) the reason was stated.
Adverse events were retrospectively registered from medical records and classified according to the National Cancer Institute Common Toxicity Criteria version 4.0. Grades 3–5 were defined as severe toxicity. Dose intensity was defined as the accumulated given dose/time unit compared to standard dose calculated from body surface area. Dose reduction was defined as cumulative given dose below the planned dose at treatment start.
For details regarding chemotherapeutic and biologic regimens, see Supplementary Table 1. All regimens were implemented before start of the inclusion period and were available during the study time period (2008–2014) [Citation23].
Statistical methods
Patients were divided into two groups according to age (50–69 or ≥70 years, since the most frequently used age cutoff dividing older from younger adults in clinical trials is 70 years [Citation22,Citation25]. Descriptive analyses of continuous variables were performed using the Wilcoxon rank sum test. Categorical variables were compared using a Chi-squared test where appropriate; otherwise Fisher’s exact test was used. Binary outcomes were analyzed by logistic regression and presented as odds ratios (ORs) and 95% confidence intervals (CIs).
PFS was defined as time from diagnosis (mCRC or non-resectable locally advanced disease) to time of progression or death from any cause. OS was defined as time from diagnosis to time of death from any cause. PFS and OS were analyzed by the Kaplan–Meier estimator; hazard ratios (HRs) and 95% CIs were estimated by Cox proportional hazards regression. CRC-specific and other cause mortality were estimated by the cumulative incidence function by the Aalen–Johansen estimator accounting for competing risk. HR for the cause-specific mortality was estimated with the Fine and Gray model, i.e., a proportional hazards model for the sub-distribution hazard accounting for competing risks. For analyses of PFS, OS, and CRC mortality, all living patients were censored at 5 years. Analyses were made with and without adjustment for PS and comorbidity using the Charlson comorbidity index to determine whether the observed differences were truly age-dependent.
The statistical software R version 3.2.2 (StataCorp, College Station, TX, USA) was used for all analyses based on complete cases, with a 5% significance level. Only complete cases were included in the analyses.
Results
A total of 654 patients were included in the DISCO study and divided into a younger group (N = 338, median 62 years (range 50–69)) and an older group of patients ≥70 years (N = 316, median 76 years (range 70–89)). In total, 97 (15%) patients were not given chemotherapy due to comorbidity, poor PS, patient refusal, or other causes, whereas 90% (N = 303) among the younger and 80% (N = 254) among the older patients received chemotherapy.
Clinical and histopathological baseline characteristics for both treated and untreated patients are given in . The following analyses are based on both treated and non-treated patients. Older patients had significantly poorer PS (p < .001) and higher comorbidity (p = .021) and received more medications (p < .001) than younger patients. They were more likely to live alone (p = .017) and to have right-sided tumors (p = .042).
Table 1. Baseline characteristics for all treated and non-treated patients.
No difference was found in KRAS mutation frequencies between the two age groups (p = .69). KRAS was more rarely analyzed in older than in younger patients (OR =3.16, 95% CI 2.11–4.74). Disease presentation at time of diagnosis (i.e., synchronous, recurrent metastatic disease, or non-resectable locally advanced disease) was similar in the two groups (p = .35), with no difference in number of metastatic sites at time of diagnosis (p = .38). Of the 90 older patients with recurrent metastatic disease, only 44 (49%) had undergone adjuvant treatment after diagnosis of primary disease, compared with 105 of the 114 younger patients (92%).
There were significant differences in baseline blood results between the two age groups, with lower hemoglobin and higher white blood cells among the older patients.
Baseline characteristics for all patients stratified by treatment or non-treatment are shown in Supplementary Table 2. There were also significant differences in baseline characteristics between treated and non-treated: non-treated patients were older (p < .001), they had poorer PS (p < .001), more comorbidity (p = .011), received more medications (p < .001), and were more likely to live alone (p = .002). The non-treated patients were also less likely to have received adjuvant after diagnosis of primary disease (p = .046). Non-treated patients had higher hemoglobin levels and lower blood cell counts.
Associations between baseline characteristics and prognosis in treated patients
PS was after adjusting for age and comorbidity associated with lower OS and PFS and higher CRC mortality (see ). After adjustment for age, comorbidity, and PS, no association was found between comorbidity, medication, BMI, sex and civil status, and treatment outcomes. Right-sided tumors were associated with lower OS (adjusted HR (aHR) = 1.54, 95% CI 1.24–1.91) and higher CRC mortality (aHR = 1.64, 95% CI 1.30–2.08) compared with left-sided tumors. Previous adjuvant treatment was associated with longer OS (aHR = 0.81, 95% CI 0.65–0.999), but there was no significant association with PFS or CRC-related mortality.
Table 2. Association between baseline characteristics, treatment, and outcomes.
Abnormal blood results (i.e., lower hemoglobin; higher leucocytes, LDH, and CEA) were associated with shorter OS and PFS and higher CRC mortality ().
Treatment
Among patients who received first-line chemotherapy, older patients were more likely to receive monotherapy instead of combination chemotherapy (adjusted OR (aOR) = 9.00, 95% CI 4.52–17.91) and not to receive additional targeting therapy with bevacizumab, cetuximab, or panitumumab (aOR = 1.89, 95% CI 1.28–2.78). Older patients with a PS of 1 and 2+ and younger patients with a PS of 2+ were more likely to receive monotherapy than those with a PS of 0 (Supplementary Table 3a). For distribution of choice of chemotherapy regimen, see Supplementary Table 4.
Older patients were less likely to receive full start dose of chemotherapy (aOR = 0.23, 95% CI 0.16–0.34). Only 40% of older patients received a full start dose compared with 77% of younger patients.
Older patients with PS 1 and younger patients with PS 1 and 2+ were less likely to receive full start dose than patients with PS 0 (Supplementary Table 3b). No significant difference was found between older patients with PS 2+ compared with those with PS 0. A total of 222 patients had primary dose reductions due to age (34%), poor PS (22%), prior chemotherapy toxicity (8%), or comorbidity (27%), which most frequently was decreased kidney function or chronic diarrhea.
Older patients received significantly lower dose intensities with 5-FU/capecitabine and oxaliplatin than younger patients (Supplementary Table 5). Older patients with PS 1 were less likely to receive dose intensity >90% than those with PS 0, but no significant difference was found for patients with PS 2. Younger patients with PS 2 also received lower dose intensities than those with PS 0 (Supplementary Table 3). No statistically significant difference was found for median dose intensities of bevacizumab and cetuximab/panitumumab between the age groups.
The proportion of chemotherapy dose reductions was similar in the two age groups (p = .16). No difference was found in number of chemotherapy cycles given, except for oxaliplatin (Supplementary Table 5).
The reasons for discontinuation of chemotherapy were similar in the two age groups: disease progression (older 51% versus younger 58%, p = .15) and toxicity or patient refusal (older 38% versus younger 31%, p = .09).
In total, 226 of 303 younger patients (75%) received subsequent lines of treatment, compared with 146 of 254 (57%) among the older patients. The likelihood of receiving later lines of treatment was significant lower for older patients also after adjusting for PS and comorbidity (aOR =0.56, 95% CI 0.39–0.82). Only 35% of the patients who received monotherapy received later lines of therapy compared with 72% in the group of patients who received combination therapy (p < .001).
Adverse events
The prevalence of severe adverse events (grades 3–5) experienced on a given dose was retrospectively noted and analyzed separately for the different treatment regimens. Small differences were observed in reported severe toxicity between older and younger patients. With regard to Capox (capecitabine and oxaliplatin)/Folfox (5-fluorouracil and oxaliplatin) regimens, older patients experienced more grade 3–4 fatigue (aOR = 4.86, 95% CI 1.27–18.57). For Folfox in combination with cetuximab, older patients developed more grade 3–4 hematologic toxicity (aOR = 11.72, 95% CI 1.54–89.01) and grade 3–5 infections (aOR = 9.24, 95% CI 1.45–58.94). Older patients were more likely to be hospitalized during treatment than younger patients, also after adjustments for PS and comorbidity, (aOR = 1.53, 95% CI 1.08–2.17). Performance status 1 compared with 0 was associated with higher toxicity regardless of regimen (p = .03). No significant association was found for PS 2 compared with PS 0. There was no difference in length of hospital stay (RR = 1.12, 95% CI 0.48–2.65) or number of hospitalizations (RR = 0.95, 95% CI 0.60–1.49) between older and younger hospitalized patients. The most frequently noted reasons for hospitalization were infections (16% for younger versus 19% for older patients), gastrointestinal symptoms (21% versus 27%), poor PS (12% versus 11%), cardiologic symptoms (7% versus 13%), and observation for or treatment of thromboembolic events (6% versus 5%), with no significant difference between the age groups. Risk of treatment-related death was higher in older patients: 18 (7.1%) older patients compared to 15 (4.3%) younger patients. Of the 18 older patients who experienced treatment-related death, eight patients (44%) died within 60 d after treatment start. Of the 15 younger patients, six patients (40%) died within 60 d. The most common cause of death was infections (in 61% of the older patients and 33% of the younger patients) and gastro-intestinal causes (17% and 33%, respectively).
Age-dependent differences in disease progression, overall survival, and CRC mortality
The older treated patients had shorter PFS than younger patients (), with a median PFS of 9.6 months (range 2 d–5 years) for the younger group and 7.5 months (range 3 d–5 years) for the older group. In patients with a PS of 0 or 1, Kaplan–Meier curves showed that older patients had a shorter PFS than younger patients. In patients with a PS of 2+, the overall survival was similar across age-groups (Supplementary Figure 1). In Cox-analysis older age was not found to be associated with shorter PFS (aOR = 1.16 95% CI (0.97–1.39) ().
Figure 2. Treatment outcomes comparing treated and non-treated patients stratified by age. (A) PFS; higher risk of progression for older compared with younger treated patients (p < .01), but after adjustments for PS and comorbidity there was no significant difference (ap = .11). (B) OS; higher risk of all-cause mortality for older compared with younger treated patients (ap < .01). No difference in non-treated patients (p = .40). (C) CRC-mortality; no age-dependent difference was found in risk of CRC-related death for treated (p = .16) or non-treated patients (p = .07). (D) Other causes of death; higher risk of non-CRC-related death in older compared with younger treated patients (ap < .01) No difference in non-treated patients (p = 0.17).
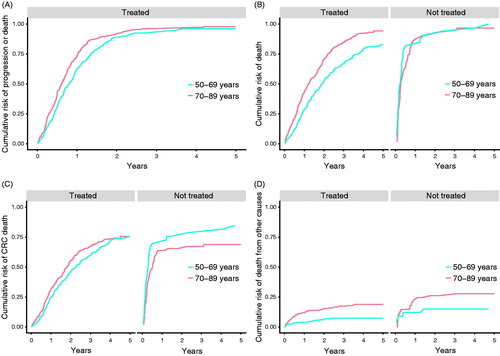
Longer OS and reduced CRC mortality were observed in both older and younger patients receiving treatment compared with patients not receiving treatment (). The Kaplan–Meier curves show longer OS in treated than in untreated older and younger patients (Supplementary Figure 2).
All-cause mortality was higher in older compared with younger patients () and older age was associated with higher all-cause mortality (). The median OS was 15.0 months (range 9 d–5 years) for the older patients and 23.0 months (range 6 d–5 years) for the younger patients. This difference was primarily caused by increased non-CRC mortality in older patients (4.7% compared with 0.9% in younger patients) (aHR = 2.32, 95% CI 1.38–3.90) (), whereas CRC mortality was more similar in the two age groups () but with a significant association between age and CRC-related mortality, also after adjustments had been undertaken ().
Associations between treatment and outcomes
Patients treated with monotherapy had shorter PFS and OS and higher CRC mortality than patients treated with combination chemotherapy (). However, after adjustments for age, PS, and comorbidity, monotherapy was only associated with decreased PFS (aHR = 1.52, 95% CI 1.16–2.00). Reduced start dose was, after adjustments, only significantly associated with shorter OS ().
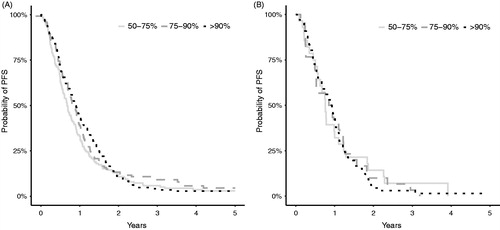
Receiving dose intensity >90% of cetuximab compared with <75% prolonged PFS (). PFS for patients given different dose intensities of the most frequently used drugs (5-FU/capecitabine and irinotecan) are shown in Kaplan–Meier curves in .
Figure 3. Kaplan–Meier curves showing PFS for patients with PS 0 and 1 given different dose intensities of chemotherapy as a monotherapy or as part of combination chemotherapy. (A) Patients given 5-FU/ capecitabine. (B) Patients given irinotecan.
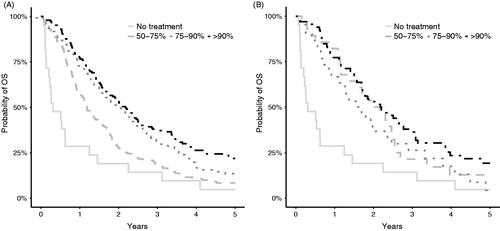
OS for patients given different dose intensities of the most frequently drugs used (5-FU/capecitabine and irinotecan) are shown in Kaplan–Meier curves in , where also non-treated patients (dose = 0) are included. OS was longer for patients given higher dose intensities of 5-FU/capecitabine, which was also seen in the association analyses, where a receiving dose intensity of >90% of 5-FU/capecitabine compared with <75% was associated with longer OS and lower CRC mortality (). There was a tendency towards longer OS with higher dose intensities of irinotecan, oxaliplatin, and cetuximab association analyses ().
Figure 4. Kaplan–Meier curves showing OS for patients with PS 0 and 1 given different dose intensities of chemotherapy as a monotherapy or as part of combination chemotherapy. Non-treated patients (dose = 0) are included. (A) Patients given 5-FU/ capecitabine. (B) Patients given irinotecan.
Patients who were hospitalized during treatment had shorter OS (aOR = 1.72, 95% CI 1.43–2.06) and PFS (aOR = 1.32, 95% CI 1.08–1.60) and higher risk of CRC mortality (aOR = 1.59, 95% CI 1.30–1.93).
Discussion
In this retrospective study of patients with mCRC referred to first-line chemotherapy, we found differences between older (≥70 years) and younger patients (50–69 years) regarding receipt of baseline examinations, treatment, choice of treatment regimens, dose intensity given, and adverse events. Older patients received less aggressive treatment and had a higher probability of receiving monotherapy instead of combination chemotherapy or addition of targeted therapy, which is in accordance with the findings of Razenberg et al. [Citation26]. Despite the less aggressive treatment, a small but significant difference in severe side effects was found in older patients than in younger patients. Older patients were also more often hospitalized and had higher treatment-related mortality than younger patients. Our findings are in accordance with those of Jackson et al. [Citation7] and Goldberg et al. [Citation27], who also found a small increased risk of adverse events among older patients. We found no difference in dose reductions or reasons for discontinuation of chemotherapy between the groups. Older patients started at reduced doses of chemotherapy and received reduced dose intensities during first-line treatment. Patients receiving a dose intensity <75% had decreased PFS (for cetuximab), and decreased OS and increased CRC mortality (for 5-FU/capecitabine), compared with patients receiving dose intensity >90%, despite a relatively small sample size. Vincent et al. [Citation28], found small differences in treatment efficacy in 221 older patients with mCRC treated with reduced doses of capecitabine 1000 mg/m2 twice daily (50% of maximal dose) compared with 750 mg/m2 twice daily. However, they found no significant difference in 1-year PFS. The reduced doses were also compared with historical data on treatment efficacy for full dose capecitabine, and a small trade off in efficacy in the form of a lower overall response rate was seen for the regimen with reduced doses. However, even if lower doses might not be as effective as higher doses they may be better than no treatment.
In the present study, higher dose intensities were not shown to prolong PFS, except for cetuximab, but there was a tendency toward longer PFS in patients treated with higher dose intensities of 5-FU/capecitabine and oxaliplatin. Due to the retrospective design, PFS for patients receiving no treatment could not be calculated because they were not included in a follow-up program. Analyses of PFS would probably have led to a too long PFS because progression might only have been recognized shortly before death in hospitalized patients. Lower CRC mortality and prolonged OS were seen with higher dose intensities of 5-FU/capecitabine, and there was a tendency toward longer OS with higher dose intensities of irinotecan, oxaliplatin, and cetuximab, but this may be caused by selection bias because older and frail patients received lower doses of chemotherapy or no treatment at all. There is still a lack of information in the literature on whether lower dose intensities in older patients with mCRC are associated with poorer treatment outcomes. This is a crucial issue because many older patients in daily clinical practice receive reduced doses, despite the lack of evidence.
Treated older patients had shorter PFS than younger patients, probably due to the less aggressive treatment. But after adjusting for PS and comorbidity, there was no difference, and we found fit older patients to be more likely to receive standard treatment, and they had a PFS like the younger patients. Also, as expected, all-cause mortality in treated patients was higher among the older patients. However, this was mainly caused by increased non-cancer mortality, likely explained by PS and comorbidities. Interestingly, CRC mortality was similar in both older and younger patients.
More intensive treatment would doubtfully have been successful in the older patients, where independence in activities of daily living is crucial for maintenance of quality of life. However, some patients might have been slightly undertreated, and 20% of the older patients referred for chemotherapy did not receive first-line treatment at all, probably due to general concerns regarding toxicity, comorbidity, and poor performance in older patients, a notion also suggested by Doat et al. [Citation29]. Correlation analyses from our study showed significantly poorer PFS for patients treated with monotherapy than for those treated with combination therapy; however, no impact was found on CRC mortality. Other studies have also reported that combination therapy provided benefits with regard to PFS and OS [Citation7,Citation8].
Poor PS was associated with decreased OS and PFS and higher CRC mortality. It might be possible to improve prognosis for this group of patients. An ongoing randomized controlled trial test whether GCA and interventions including rehabilitation can improve health status in frail older patients with CRC and enhance the number of patients completing scheduled adjuvant or first-line chemotherapy [Citation30].
Other factors may have had prognostic influence; older patients were more likely to have a right-sided tumor, which is associated with poorer prognosis [Citation31], and poorer OS was also seen in the present study in these patients. Furthermore, more younger than older patients received later lines of treatment, which could impact survival and CRC mortality, but not PFS.
Recurrence after adjuvant chemotherapy is usually associated with poorer prognosis, based on the hypothesis regarding the development of chemotherapy-resistant tumor, e.g., lower survival rate after recurrence in older patients who have received adjuvant 5-FU and oxaliplatin compared with 5-FU alone [Citation32]. But for patients with recurrent disease in the present study, previous adjuvant chemotherapy was found to be associated with longer OS. The reason for this finding might be selection bias, where only fit older patients had been offered adjuvant chemotherapy at the time the primary diagnosis was made. Their physical fitness also predicted better prognosis in the palliative setting. We found no negative impact of prior adjuvant treatment on PFS or CRC mortality, perhaps because of the small sample size.
A few baseline blood parameters, lower hemoglobin and higher white blood cells, were significantly associated with poorer outcomes in older patients. Serum levels of LDH and CEA were similar in the two groups, which corroborates that stage of disease was similar, and the differences in other baseline blood results are probably a result of the higher comorbidity seen in the older patients. Higher hemoglobin levels and lower white blood cell counts was surprisingly seen in non-treated than in treated patients, without any good explanation.
We found that older patients were less likely to have undergone standard baseline KRAS evaluation, which might have led to a lower likelihood of receiving additional cetuximab or panitumumab. However, many older patients with missing KRAS status were probably not considered for biological treatment initially, perhaps because of concerns regarding toxicity and lack of evidence for the efficacy of anti-EGFR in the older population [Citation15,Citation33].
Our retrospective single-center DISCO study has several strengths. One physician collected and interpreted all data to minimize interpersonal variation. We assessed the cause of death, which is crucial when evaluating the effect of chemotherapy in older patients because they have a higher non-cancer mortality. To our knowledge, our study is the first to evaluate the impact of different dose intensities on treatment outcomes in older patients with mCRC.
Study limitations include the retrospective design including retrospective registering of adverse events, which should be analyzed with caution. A relatively small sample size, especially in the group of patients with PS 2+, probably explains why associations between PS2+ and increased risk of adverse events, risk of reduced start dose and low dose intensity (Supplementary Table 3) were not found. Analyzing multiple tests on the same data set increased the chance of obtaining at least one invalid result. However, despite the small sample size, we showed statistically significant differences between younger and older patients, with multiple results pointing in the same direction.
Although both treated and non-treated patients were eligible for the study, there is likely a selection bias because the frail patients were not referred to treatment. Unfortunately, we did not have data on the number of patients diagnosed with metastatic disease during the period. Such an investigation would be time-consuming and was not the purpose of this study.
Data were collected between 2008 and 2014, and analysis of some clinical biomarkers (e.g., tumor characteristics and KRAS and BRAF analysis) was not implemented in daily routines during the first study period. In this study, no adjustments for year of treatment were made in the analyses of choice of regimens. It is unknown whether treatment patterns or outcomes changed over time.
The results from our retrospective study are only hypothesis-generating, and future research is needed with focus on CGA and exercise to optimize poor PS and thereby prognosis, but also studies investigating the potential benefit of different dose intensities and regimens such as the Nordic 9 protocol, comparing full dose monotherapy with the 5-FU prodrug S1 with reduced doses of combination chemotherapy with S1 and oxaliplatin [Citation34], but also studies on anti-EGFR therapy. Several randomized trials that have included older and frail patients have been performed [Citation10–12]. However, patient inclusion may be challenging because an oncologist could be reluctant to randomize older patients to various regimens or intensities without considering physical fitness, a factor responsible for poor accrual in the NCCTG N0949 trial [Citation35]. Trial design is crucial for enrollment of unfit older patients, and CGA before randomization may help define treatment strategy in order to minimize toxicity for vulnerable patients and to secure adequate treatment for fit older patients [Citation36].
In conclusion, in our study, older patients received less aggressive first-line chemotherapy compared with younger patients, also after adjustment for PS and comorbidity. Despite this less aggressive treatment, the older patients experienced more side effects, hospitalizations, and treatment-related mortality. Decreased dose intensity of 5FU/capecitabine was associated with poorer OS and higher CRC mortality. The general trend throughout this study was the emergence of significant age-dependent differences in outcomes PFS, OS, and CRC mortality, which disappeared after adjustments for poor PS and comorbidity, implying that patients with poor PS and comorbidity, independent of age, have poorer treatment outcomes. Future research should focus on the possibility of improving PS in this group of patients, preferably in association with geriatric oncology care programs.
Ethical approval
This is a retrospective study. Collection and storing of data in a database was approved by the Danish Health Authorities (3-3013-990/1) and the Danish Data Protection Agency (HEH-2015-015 and I-Suite no: 03531).
| Abbreviations | ||
| aOR | = | adjusted odds ratio |
| EGFR | = | epidermal growth factor receptor |
| Capox | = | capecitabine and oxaliplatin |
| CGA | = | comprehensive geriatric assessment |
| CI | = | confidence interval |
| CRC | = | colorectal cancer |
| Folfox | = | 5-fluorouracil and oxaliplatin |
| 5-FU | = | 5-fluorouracil |
| HR | = | hazard ratio |
| mCRC | = | metastatic colorectal cancer |
| MSI | = | microsatellite instability |
| OR | = | odds ratio |
| OS | = | overall survival |
| PFS | = | progression-free survival |
| PS | = | performance status |
Supplemental Material
Download Zip (732.2 KB)Disclosure statement
The authors have declared no conflicts of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
- Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208.
- Piessevaux H, Buyse M, Schlichting M. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31:3764–3775.
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. JCO. 2009;27:3677–3683.
- Braendegaard Winther S, Baatrup G, Pfeiffer P, et al. Trends in colorectal cancer in the elderly in Denmark, 1980–2012. Acta Oncol. 2016;55(Suppl 1):29–39.
- Hoeben KW, van Steenbergen LN, van de Wouw AJ, et al. Treatment and complications in elderly stage III colon cancer patients in the Netherlands. Ann Oncol. 2013;24:974–979.
- Jackson NA, Barrueco J, Soufi-Mahjoubi R, et al. Comparing safety and efficacy of first-line irinotecan/fluoropyrimidine combinations in elderly versus nonelderly patients with metastatic colorectal cancer: findings from the bolus, infusional, or capecitabine with camptostar-celecoxib study. Cancer. 2009;115:2617–2629.
- Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. JCO. 2008;26:1443–1451.
- Bosse D, Vickers M, Lemay F, et al. Palliative chemotherapy for patients 70 years of age and older with metastatic colorectal cancer: a single-centre experience. Curr Oncol. 2015;22:349–356.
- Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol. 2016;27:121–127.
- Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–1759.
- Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–1085.
- Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–743.
- Rouyer M, Fourrier-Reglat A, Smith D, et al. Effectiveness and safety of first-line bevacizumab plus FOLFIRI in elderly patients with metastatic colorectal cancer: results of the ETNA observational cohort. J Geriatr Oncol. 2016;7:187–194.
- Jehn CF, Boning L, Kroning H, et al. Cetuximab-based therapy in elderly comorbid patients with metastatic colorectal cancer. Br J Cancer. 2012;106:274–278.
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Jco. 2011;29:3457–3465.
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806.
- Kordatou Z, Kountourakis P, Papamichael D. Treatment of older patients with colorectal cancer: a perspective review. Ther Adv Med Oncol. 2014;6:128–140.
- Power DG, Lichtman SM. Chemotherapy for the elderly patient with colorectal cancer. Cancer J. 2010;16:241–252.
- Lund CM, Nielsen D, Dehlendorff C, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colorectal cancer: the ACCORE study. ESMO Open. 2016;1:e000087.
- Lieu CH, Renfro LA, de Gramont A, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD clinical trials program. J Clin Oncol. 2014: 32:2975–2984.
- Papamichael D, Audisio RA, Glimelius B, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463–476.
- Vincent MD, Breader D, Cripps MC, et al. Phase I/II trial of dose-reduced capecitabine in elderly patients with advanced colorectal cancer. Curr Oncol. 2017;24:261–e268.
- O'Connell JB, Maggard MA, Livingston EH, et al. Colorectal cancer in the young. Am J Surg. 2004;187:343–348.
- Hotta K, Ueoka H, Kiura K, et al. An overview of 48 elderly-specific clinical trials of systemic chemotherapy for advanced non-small cell lung cancer. Lung Cancer. 2004;46:61–76.
- Razenberg LG, van Erning FN, Pruijt HF, et al. The impact of age on first-line systemic therapy in patients with metachronous metastases from colorectal cancer. J Geriatr Oncol. 2017;8:37–43.
- Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. JCO. 2006;24:4085–4091.
- Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55:241–252.
- Doat S, Thiebaut A, Samson S, et al. Elderly patients with colorectal cancer: treatment modalities and survival in France. National data from the ThInDiT cohort study. Eur J Cancer. 2014;50:1276–1283.
- Lund CM, Vistisen KK, Dehlendorff C, et al. The effect of geriatric intervention in frail elderly patients receiving chemotherapy for colorectal cancer: a randomized trial (GERICO). BMC Cancer. 2017;17:448.
- Gervaz P, Usel M, Rapiti E, et al. Right colon cancer: left behind. Eur J Surg Oncol. 2016;42:1343–1349.
- Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. JCO. 2012;30:3353–3360.
- Aparicio T, Pamoukdjian F, Quero L, et al. Colorectal cancer care in elderly patients: unsolved issues. Dig Liver Dis. 2016;48:1112–1118.
- Winther SB, Osterlund P, Berglund A, et al. Randomized study comparing full dose monotherapy (S-1 followed by irinotecan) and reduced dose combination therapy (S-1/oxaliplatin followed by S-1/irinotecan) as initial therapy for older patients with metastatic colorectal cancer: NORDIC 9. BMC Cancer. 2017;17:548.
- McCleary NJ, Hubbard J, Mahoney MR, et al. Challenges of conducting a prospective clinical trial for older patients: lessons learned from NCCTG N0949 (alliance). J Geriatr Oncol. 2018;9:24–31.
- Kelly CM, Power DG, Lichtman SM. Targeted therapy in older patients with solid tumors. J Clin Oncol. 2014;32:2635–2646.