Abstract
Background/Purpose: To investigate prognostic factors for death within 6 months of stereotactic body radiotherapy (SBRT) for patients with peripheral early-stage non-small cell lung cancer (NSCLC).
Materials and Methods: This analysis included 586 NSCLC patients with peripheral tumors treated with SBRT. Potential patient and tumor prognostic factors, including the Charlson Comorbidity Index (CCI) and Cumulative Illness Rating Scale (CIRS), were analyzed by logistic regression analysis for association with early mortality (death <6 months after SBRT). Additionally, CCI and CIRS were compared with respect to their predictive ability for early mortality by comparing multivariate models with each comorbidity index, and assessing their respective discriminatory abilities (C-index).
Results: A total of 36 patients (6.1%) died within 6 months of the start of SBRT. With a median follow-up of 25 months, 3-year overall survival was 54%. CIRS and tumor diameter were significant predictors of early mortality on multivariate analysis (p = .001). Patients with a CIRS score of 8 or higher and a tumor diameter over 3 cm had a 6-month survival of 70% versus 97% for those lacking these two features (p < .001). CCI was not predictive for early mortality on univariate nor multivariate analysis; the model containing CCI had a C-index of 0.65 versus 0.70 for the model containing CIRS.
Conclusion: CIRS and tumor diameter predict for early-mortality in peripheral early-stage NSCLC treated with SBRT. CIRS may be a more useful comorbidity index than CCI in this population when assessing short-term life expectancy.
Introduction
Stereotactic body radiotherapy (SBRT) provides a curative-intent treatment option for patients with inoperable early-stage non-small cell lung cancer (NSCLC). In years preceding the advent of SBRT, the majority of patients with severe comorbidities or advanced age were managed with palliative treatment or supportive care alone [Citation1]. Studies in the Netherlands have demonstrated increased utilization of curative-intent treatment since the introduction of SBRT, with a corresponding improvement in survival rates [Citation2].
Due to a high burden of comorbid illness in the lung SBRT population, rates of overall survival lag behind those for cancer-specific survival [Citation3,Citation4]. Previously, we reported that more than two-thirds of deaths in a population of early-stage NSCLC patients were from non-cancer causes [Citation5]. Indeed, a proportion of patients will not benefit from SBRT due to competing mortality and limited life expectancy. These patients may be better served with a supportive care approach, sparing patients the inconvenience and potential cost of SBRT, and utilizing the resource-intensive treatment more judiciously. As more reports emerge describing the favorable toxicity profile in octogenarians [Citation6] and patients with severe COPD [Citation7,Citation8], the decision between SBRT and best supportive care may become an increasingly frequent clinical dilemma.
Short-term survival outcomes in this population, however, remain largely unstudied [Citation3,Citation9]. Although recent consensus guidelines from the European Society for Radiotherapy and Oncology (ESTRO) identify short estimated life expectancy as a contraindication for treatment [Citation10], prognostic factors for short-term survival have not been elucidated. The primary objective of this study was to identify factors associated with early mortality, defined as death within 6 months of SBRT, in order to assist patients and clinicians with weighing the different management options for early-stage NSCLC. Secondarily, we aimed to compare two well-known comorbidity indices, the Charlson Comorbidity Index (CCI) and the Cumulative Illness Rating Scale (CIRS), with respect to their ability to predict early mortality.
Materials and methods
Patients
Consecutive peripheral early-stage NSCLC patients treated with 4-dimensional SBRT at the Department of Radiotherapy at Erasmus MC between August 2005 and January 2017 were identified. Patients lacking histologic confirmation were recommended for SBRT based on positron emission tomography (PET)-CT scan findings and multidisciplinary tumor board review. Details regarding treatment protocol have been previously described [Citation5,Citation11]. In brief, the gross tumor volume plus a 5 mm margin to account for microscopic tumor extension and geometric positional uncertainty was irradiated, typically in three fractions, on the Cyberknife radiosurgery system (Accuray Inc., Sunnyvale, CA). The following exclusion criteria were applied: central location (within 2 cm of the proximal bronchial tree), synchronous intrapulmonary lesions, histology other than NSCLC, delivered biologically effective dose (BED) < 100 Gy assuming an α/β ratio of 10, and follow-up time less than 6 months from the start of radiotherapy. Tumor staging was performed based on PET-CT scan, while mediastinal staging was based on PET-CT, mediastinoscopy, and/or endobronchial ultrasound (EBUS). For the present study, patients were re-staged according to American Joint Committee on Cancer (AJCC) 8th edition. Global Initiative for Chronic Obstructive Lung Disease (GOLD) scores were obtained [Citation12] and were not reclassified to reflect 2017 criteria, since these incorporate comprehensive symptom assessment with validated questionnaires [Citation13] and these data were not available retrospectively. Clinical and imaging follow-up was performed as previously described [Citation5].
Comorbidity was assessed retrospectively using both CCI and CIRS, based on the electronic medical record. The CCI is a widely used metric to assess comorbidity and consists of 19 clinical conditions weighted for the relative risk of death. It was first developed in 1987 based on the 1-year mortality of patients admitted to a medical hospital service for a variety of reasons, and externally validated in a cohort of breast cancer patients [Citation14]. The CIRS was developed in 1968 and scores the severity of disease in 13 organ systems from 0 (no problem) to 4 (extremely severe) [Citation15]. Both CCI and CIRS have been used to study comorbidity burden in a variety of oncologic populations with reliable results and good interrater reliability [Citation16,Citation17]. For the present study, CCI and CIRS were scored as previously described [Citation14,Citation15].
Statistics
The primary endpoint was early mortality, defined as death within 6 months of the start of SBRT. Univariable analysis of potential prognostic factors for early mortality was performed using binary logistic regression analysis. Covariates included age, gender, Karnofsky Performance Status (KPS), operability, CCI, CIRS, smoking status (current/former versus never), GOLD score, previous malignancy, previous lung cancer, maximum axial tumor diameter, and lower lobe location. Tumor diameter was dichotomized based on size criteria for AJCC TNM T-staging (≤3 cm versus >3 cm). Other continuous variables were dichotomized based on the median value (≤median versus >median). CIRS and CCI were additionally analyzed as continuous variables. Variables with a p-value ≤.2 on univariable analysis were analyzed in a multivariate logistic regression analysis using the forward selection method.
To compare the predictive ability of CCI and CIRS, two separate multivariate models were constructed and the discriminatory ability of each model assessed by the C-index. Each multivariable model was constructed by including all co-variates with a p-value ≤.2 on univariable analysis, plus the comorbidity index in question. Overall survival (OS) was estimated using the Kaplan–Meier method. Log rank tests assessed for differences in OS when stratifying by prognostic factors selected by the model building procedure. All statistical analyses were performed in SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The study protocol was approved by the Medical Ethical Committee of the Erasmus Medical Center (MEC2016-729).
Results
A total of 586 patients were included in analysis. Median age was 75 years (range 44–91) and 93.2% of patients were deemed medically or surgically inoperable upon multidisciplinary tumor board review. Additional baseline patient and tumor characteristics are provided in . The median CCI score was 3 (range 1–14) while the median CIRS was 5 (range 0–15). Respiratory disease was the most frequent CIRS comorbidity (80.0%) followed by vascular and cardiac (49.8% and 44.5%, respectively) ().
Table 1. Baseline clinical and treatment characteristics of 586 patients with peripheral early stage non-small cell lung cancer treated with stereotactic body radiotherapy.
Table 2. The frequency of comorbidities within the 14 organ systems comprising the Cumulative Illness Rating Scale.
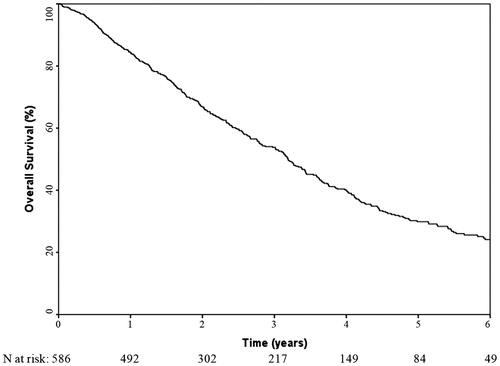
A total of 36 patients (6.1%) died within 6 months of the start of SBRT. Among these patients, eight had experienced disease progression: four had distant metastases alone, three had regional (mediastinal lymph nodes) and distant recurrences, and 1 had local, regional, and distant recurrences. The median follow-up time for surviving patients was 26.1 months (range 10.4–124.6). The median overall survival was 38.3 months (95% confidence interval 34.2–42.3). Rates of 6-month, 1-year, 3-year and 5-year overall survival were 93.7%, 84.5%, 53.8% and 29.9%, respectively ().
Figure 1. Kaplan–Meier curve showing the overall survival of the 586 early-stage lung cancer patients treated with stereotactic body radiotherapy.
Only tumor diameter (>3 cm vs ≤3 cm) and CIRS score were significantly associated with early mortality in univariable analysis (). In multivariable analysis, both tumor diameter (odds ratio [OR] 3.45, 95% CI 1.72–6.92; p < .001) and CIRS score (OR 1.27, 95% CI 1.10–1.45; p < .001) were significant predictors of early mortality. Patients with both a CIRS score of 8 or more (the highest quartile of CIRS score in the population) and a tumor diameter >3 cm had a Kaplan–Meier estimated 6 month OS of 70%, compared to 97% in patients with neither of these adverse prognostic features present (p < .001) (); 1-year OS rates were 63% and 90%, respectively. The C-index for the multivariable model containing CIRS as a co-variate was 0.70 versus 0.65 for the multivariable model containing CCI.
Figure 2. Kaplan–Meier curve showing the overall survival of the 586 early-stage lung cancer patients treated with stereotactic body radiotherapy, stratified by Cumulative Illness Rating Scale (CIRS) score and tumor diameter. (Log rank p < .001).
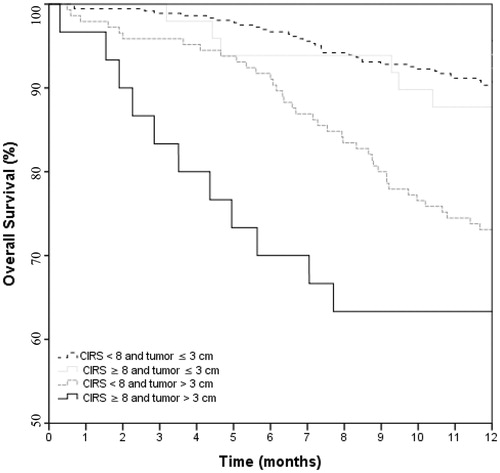
Table 3. Variables associated with early mortality (death within 6 months of stereotactic body radiotherapy).
Discussion
Short-term survival outcomes are an important and under-studied endpoint in early-stage NSCLC. Here, we have identified CIRS score, as well as tumor diameter, as important prognostic factors for early mortality. Furthermore, we have provided a quantitative assessment of the two factors on survival time, in order to assist patients and clinicians in making informed treatment decisions.
The importance of CIRS score for short-term survival is not surprising, given the high burden of comorbidity in the inoperable NSCLC population. CCI, however, was not prognostic for early mortality. This is an important finding, given that CCI is currently the most frequently used comorbidity metric in NSCLC, and may influence the therapeutic choice between definitive treatment and supportive care [Citation18]. Indeed, recent lung SBRT practice guidelines mention CCI as a comorbidity measure when considering appropriate patient selection for treatment [Citation10]. Additionally, studies comparing surgical and SBRT outcomes commonly utilize CCI for propensity-score matching [Citation19–21]. CIRS, however, may be a more appropriate metric for these purposes. While previous studies have found CCI to predict for OS in the lung SBRT population [Citation22–24], one study which examined death within 6 months of treatment as an endpoint found that while CCI was predictive of OS, it was not significantly associated with early mortality [Citation9]. Of note, CIRS was not included as a potential prognostic factor.
This discrepancy between CIRS and CCI for early mortality may be due to several factors. By grading diseases in each organ system from zero to four, CIRS is more sensitive to disease severity, which may be an important determinant of short-term survival. One study reported CIRS, and not CCI, was associated with length of hospital admission, suggesting CIRS may detect acute comorbidities that CCI does not [Citation25]. Indeed, the predictive ability of CCI may progressively decline with shorter survival endpoints [Citation16]. Conversely, the greater emphasis of CCI on chronic conditions may make it a more suitable metric for long term survival outcomes. Furthermore, CIRS may capture additional comorbidities that CCI does not. Extermann et al. [Citation17] reported the prevalence of comorbidity in an elderly cancer population to be 36% with CCI compared to 94% with CIRS. The finding that CCI may underestimate comorbidity prevalence was also reported in a study on prognostic factors for OS in stage I NSCLC [Citation26]. While CIRS was prognostic for both patients treated with conventional RT or surgery, CCI was only prognostic in the radiotherapy cohort, which consisted of patients with a higher comorbidity burden.
The importance of tumor diameter as a prognostic factor for early mortality is consistent with previous studies examining OS. Kopek et al. [Citation22] reported that lung SBRT patients with T2 tumors had poorer OS than those with T1 tumors. Other studies have reported similar findings [Citation22,Citation27–29]. It is perhaps surprising, however, that tumor diameter is strongly associated with early mortality. Indeed, it is notable that 8 patients with early mortality had developed recurrent disease, and in all cases, this included distant metastases. This finding highlights the early metastatic potential of NSCLC, and suggests the need to for improved detection of occult metastases at the time of diagnosis. Of note, all patients in the present study had undergone staging with PET-CT scan.
The factors which lacked association with early mortality warrant comment. Advanced age is commonly perceived as an adverse prognostic feature, and elderly patients with lung cancer may be less likely to receive active treatment than younger patients after controlling for other adverse features [Citation30,Citation31]. Similarly, GOLD score was not associated with early mortality, despite the known impact of COPD severity on OS [Citation32]. Given the demonstrated safety of SBRT for these patients [Citation6–8], it is reassuring that they do not experience poor short-term survival; age and COPD severity should not preclude curative-intent treatment.
Death within 6 months of SBRT was defined as early mortality, as this represents the scenario where patients do not live long enough to benefit from treatment. The appropriate definition of early mortality is dependent on the natural history of untreated NSCLC, and the timeframe in which cancer-related morbidity and mortality commonly occur. A systematic review reported a mean survival of 11.94 months (95% CI 10.07–13.8) for untreated early stage NSCLC [Citation33]. We acknowledge that the minimum life expectancy to warrant definitive treatment is somewhat controversial [Citation10], however, we chose the 6-month time point as a life expectancy for which most patients and clinicians would not favor radical treatment.
Previous studies on short-term survival in lung SBRT patients have focused almost exclusively on 30- and 90-day mortality, in order to facilitate comparison with surgical perioperative mortality [Citation19]. In this context, a comprehensive assessment of prognostic factors for short-term morality in lung SBRT has not been conducted. One previous study did examine death within 6 months of SBRT as an endpoint [Citation9]. Interestingly, only Eastern Cooperative Oncology Group (ECOG) performance status was associated with early mortality, although CIRS and tumor diameter were not included as covariates. It is surprising that performance status was not predictive of early mortality in the present study. One possible explanation is the low number of patients with very poor performance status (only 10 patients with KPS 50). It is notable, however, that other studies on prognostic factors in lung SBRT patients have found performance status to lack prognostic significance [Citation22,Citation27], although conflicting reports exist [Citation23,Citation26,Citation28].
Limitations of the study include its retrospective nature, and the small number of events observed. Retrospective scoring of CIRS and CCI may not have captured all comorbidities. However, detailed clinical notes were available for all patients, and the majority of clinically relevant comorbidities were likely documented. Additionally, there were a small number of events observed. It is encouraging that only 6% of patients experienced death within 6 months of SBRT. However, low event number may have reduced statistical power for detecting potential prognostic factors. Of note, patients in the present study had been deemed suitable SBRT candidates after tumor board review. Investigating the survival times and prognostic factors for patients who are not referred for SBRT due to short anticipated life expectancy would yield valuable insights. An additional limitation of the analysis is that the prognostic factors identified cannot in isolation identify patient groups with very poor short-term survival; even patients with tumor diameter greater than 3 cm and CIRS scores of 8 or higher had a 6-month OS of 70%. This relatively high 6-month survival is an important observation, highlighting that patients with high CIRS score and large tumor diameter should not be excluded from SBRT on the basis of these characteristics alone. Whether additional adverse prognostic features may be identified which in combination reliably predict for very poor short term survival such that forgoing SBRT is warranted remains to be elucidated. Finally, we were unable to report the cause of death, as this information was not available for the majority of patients. Hence, short-term cancer-specific survival could not be assessed, nor treatment-related mortality. However, it is reassuring that we previously observed no grade 4–5 toxicity in peripheral early stage lung cancer patients treated with this regimen [Citation5]. Further study into the cause of early mortality might yield valuable insight, such as whether comorbidities captured on CIRS and their severity are related to the specific cause of death. Nevertheless, the survival estimates here, as well as the identification of CIRS as an important determinant of short-term survival, provide useful information for patients and clinicians when discussing the cost-benefit analysis for definitive treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012;23:2743–2747.
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. JCO. 2010;28:5153–5159.
- Murray P, Franks K, Hanna GG. A systematic review of outcomes following stereotactic ablative radiotherapy in the treatment of early-stage primary lung cancer. BJR. 2017;90:20160732.
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014;90:603–611.
- van der Voort van Zyp NC, Prévost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol. 2009;91:296–300.
- Cassidy RJ, Patel PR, Zhang X, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer in patients 80 years and older: a multi-center analysis. Clin Lung Cancer. 2017;18:551–558.
- Baumann P, Nyman J, Hoyer M, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer - a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol. 2008;88:359–367.
- Takeda A, Enomoto T, Sanuki N, et al. Reassessment of declines in pulmonary function ≥1 year after stereotactic body radiotherapy. Chest. 2013;143:130–137.
- Klement RJ, Belderbos J, Grills I, et al. Prediction of early death in patients with early-stage NSCLC-can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol. 2016;11:1132–1139.
- Guckenberger M, Andratschke N, Dieckmann K, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124:11–17.
- Nuyttens JJ, van de Pol M. The Cyber Knife radiosurgery system for lung cancer. Expert Rev Med Devices. 2012;9:465–475.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626.
- Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181–200.
- Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36:453–471.
- Gould MK, Munoz-Plaza CE, Hahn EE, et al. comorbidity profiles and their effect on treatment selection and survival among patients with lung cancer. Annals ATS. 2017;14:1571–1580.
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-treatment mortality after surgery and stereotactic body radiotherapy for early-stage non-small-cell lung cancer. J Clin Oncol. 2018;36:642–651.
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer. 2013;119:2683–2691.
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: a propensity score matching analysis. Eur J Cancer. 2014;50:2932–2938.
- Kopek N, Paludan M, Petersen J, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009;93:402–407.
- Louie AV, Haasbeek CJ, Mokhles S, et al. Predicting overall survival after stereotactic ablative radiation therapy in early-stage lung cancer: development and external validation of the amsterdam prognostic model. Int J Radiat Oncol Biol Phys. 2015;93:82–90.
- Rosen JE, Keshava HB, Yao X, et al. The natural history of operable non-small cell lung cancer in the National Cancer Database. Ann Thorac Surg. 2016;101:1850–1855.
- Rochon PA, Katz JN, Morrow LA, et al. Comorbid illness is associated with survival and length of hospital stay in patients with chronic disability. A prospective comparison of three comorbidity indices. Med Care. 1996;34:1093–1101.
- Firat S, Bousamra M, Gore E, et al. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:1047–1057.
- Matsuo Y, Shibuya K, Nagata Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1104–1111.
- Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:1064–1070.
- Dunlap NE, Larner JM, Read PW, et al. Size matters: a comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT). J Thorac Cardiovasc Surg. 2010;140:583–589.
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. JCO. 2007;25:5570–5577.
- Blanco JA, Toste IS, Alvarez RF, et al. Age, comorbidity, treatment decision and prognosis in lung cancer. Age Ageing. 2008;37:715–718.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931.
- Wao H, Mhaskar R, Kumar A, et al. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev. 2013;2:10.
