Abstract
Introduction: Recently, new targeted agents have been developed, which can prolong the effect of endocrine treatment (ET) by targeting resistance pathways in HR+/HER2− advanced breast cancer. This review examines available studies of everolimus, an mTOR inhibitor, and the CDK 4/6 inhibitors ribociclib, palbociclib and abemaciclib in terms of efficacy, tolerability and safety.
Material and methods: A systematic literature search was performed in Pubmed. Evaluation of the quality of the identified studies was based on selected elements from the GRADE guidelines.
Results: The literature search yielded eight randomized trials that all presented a significant increase in the progression free survival (PFS)/time to progression (TTP) for the targeted agents plus ET vs ET only. The improvement was evident as first-line therapy with an increase in PFS of 10–11 months when adding a CDK4/6 inhibitor to ET, as well as in patients previously treated for metastatic disease, with an increase of 5–6 months. The common adverse events (AEs) of the CDK 4/6 inhibitors were due to myelosuppression. In addition, abemaciclib was associated with liver toxicity and diarrhea, and ribociclib with liver toxicity and QTcF prolongation. The most common grade 3/4 AE of everolimus was stomatitis. The majority (five) of the trials had no serious limitations, and thus the quality of evidence was high.
Discussion: The new targeted agents are all associated with an improvement of the PFS with an acceptable tolerability, and they should be offered to women with advanced HR+/HER2− breast cancer both as first-line therapy as well as among patients previously treated in metastatic regimens. However, further data regarding the impact on overall survival are required to evaluate the full benefit for patients. Price and differences in AEs could become substantial arguments for the choice of therapy for the individual patient.
Introduction
Metastatic breast cancer (MBC) has a five-year survival rate of only 26%, for which reason it is essential to develop more effective therapies [Citation1]. The treatment of MBC is not curative but palliative, focusing on optimizing survival time and quality of life and limiting adverse events [Citation2]. Almost 75% of all breast cancer cases are hormone receptor positive (HR+), and most are human epidermal growth factor receptor 2 negative (HER2−) [Citation1]. The HR+/HER2− MBC is usually managed with highly tolerable endocrine therapy (ET) consisting of aromatase inhibitors or estrogen receptor blockers. However, a proportion of MBCs does not respond to ET due to intrinsic resistance and the majority will eventually develop disease progression because of acquired resistance [Citation3,Citation4]. For first-line ET, the median progression free survival (PFS) varies from 6 to 17 months [Citation5–10].
Recently, biologically targeted agents have been developed, which aim to work synergistically with ET and thereby improve the PFS. This review will evaluate available randomized trials on the mammalian target of rapamycin (mTOR) inhibitor, everolimus, and the cyclin-dependent kinase (CDK) 4/6 inhibitors, ribociclib, palbociclib and abemaciclib in combination with ETs in HR+/HER2− MBC regarding efficacy, tolerability and safety.
Mechanism
In the HR+/HER2− BC cell, acquired resistance can be caused by various mechanisms such as alterations in the estrogen receptor (ER) signaling pathway and by up-regulation of alternative molecular signaling pathways [Citation11].
Everolimus is an inhibitor of the mTOR protein in the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway, downstream of membrane tyrosine kinase receptors such as epidermal growth factor receptor (EGFR), HER2 and insulin-like growth factor 1 receptor (IGF-1R), which regulate ER signaling [Citation1]. One mechanism of resistance is through crosstalk between the growth factor receptors and ER, which activates downstream pathways including the PI3K pathway [Citation12]. Another possible mechanism is phosphorylation of the ER by an mTOR substrate named S6 kinase 1, leading to receptor activation independent of hormonal stimulation [Citation12]. The aberrant activation of the PI3K/AKT/mTOR pathway was demonstrated in preclinical studies on estrogen deprived BC cell lines (mimicking aromatase inhibitor treatment), which found an increased phosphorylation of the substrates of mTOR and PI3K, and furthermore, that inhibition of the mTOR and PI3K led to apoptosis [Citation13–15].
Ribociclib, palbociclib and abemaciclib are selective inhibitors of cyclin-dependent kinase (CDK) 4 and 6 [Citation1]. When cyclin D binds to CDK 4/6, the complex phosphorylates and hereby deactivates the retinoblastoma protein (Rb), a tumor suppressor protein, resulting in cell cycle progression from G1 to the S phase and thus cell division [Citation16,Citation17]. Dysregulations in the cyclin D-CDK4/6-Rb-E2F pathway, such as overexpression of cyclins E1 and D1 and CDK 6, are common in HR + BC and associated with endocrine resistance [Citation18–20]. Preclinical studies of abemaciclib and palbociclib have shown that blocking the Rb phosphorylation causes G0/G1 cell cycle arrest, leading to restored sensitivity to ET in previously resistant cell lines [Citation20–22].
Material and methods
Literature search
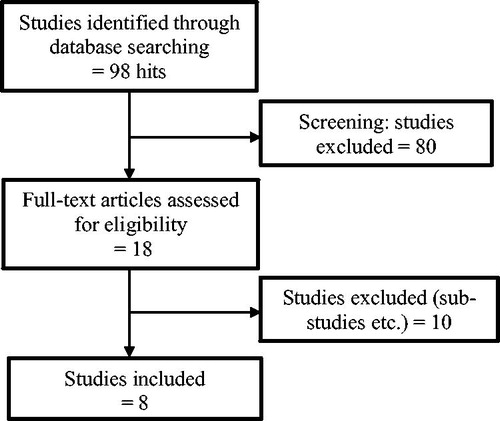
A Pubmed search on the 2 November 2017, with the search terms ‘(everolimus OR abemaciclib OR ribociclib OR palbociclib) AND (metastatic OR advanced) AND (“Breast Neoplasms”[Mesh] OR “breast cancer”) AND randomized’, yielded 98 results. From the title and abstract, phase II and III randomized controlled trials (RCT) were selected in accordance with the following inclusion criteria: treatment regimens of either everolimus, abemaciclib, ribociclib or palbociclib in combination with ET compared to ET only in HER2 negative advanced disease. Only English-language publications were reviewed. Publications were excluded due to: HER2 positive or only localized disease, adjuvant and neo-adjuvant treatment or single-agent therapy. The selected publications were final or complete analyses of the trials ().
Figure 1. Literature search and study selection. The literature search yielded 98 articles. A screening of the main titles and abstracts resulted in 18 studies, where the full text was assessed for eligibility. Further ten studies were excluded due to our exclusion criteria or because some were sub-studies of already included articles, leading to the final eight included studies.
Quality assessment
Evaluation of the quality of the identified studies was based on selected elements from the GRADE guidelines [Citation23]. In the GRADE approach, RCTs start as high-quality evidence, but can be downgraded if the studies suffer from a high risk of bias [Citation24]. The following elements were evaluated for the primary efficacy outcome; randomization of patients (risk of selection bias), blinding (risk of performance bias), lost to follow-up and whether the results were analyzed in accordance with the intention to treat (ITT) principle or per protocol. Additionally, unequal attritions and whether outcomes were assessed by blinded investigators (risk of detection bias) were evaluated.
Results
Study information and patient populations
The literature search yielded eight RCTs () fulfilling our inclusion criteria. Everolimus was evaluated in Bachelot et al. and BOLERO-2 [Citation25,Citation26], ribociclib in MONALEESA-2 [Citation27], palbociclib in PALOMA-1, -2 and -3 (final analysis) [Citation28–30] and abemaciclib in MONARCH 2 and -3 [Citation31,Citation32]. In these trials, the study agent was either combined with an anti-estrogen (tamoxifen [Citation25] or fulvestrant [Citation30,Citation31]) or an aromatase inhibitor (exemestane [Citation26] or letrozole [Citation27–29,Citation32]). Patients in four studies received the study treatments as first-line metastatic treatment [Citation27–29,Citation32], while all patients in two trials were previously treated for metastatic disease [Citation25,Citation30]. In two trials, the study populations were mixed; in BOLERO-2, 21% received the study treatment as first-line, while 79% were previously treated in metastatic regimens [Citation26], and in MONARCH 2, 38% were previously treated for metastatic disease [Citation31]. Almost all patients had previously received adjuvant or neo-adjuvant ET. Six studies did only include postmenopausal women [Citation25–29,Citation32], while two (MONACH 2 and 3) also included pre- or peri-menopausal women, 17% and 21%, respectively [Citation30,Citation31]. In the first-line trials, patients with de novo metastatic disease was included and accounted for 34% to 49% of the study population [Citation27–29,Citation32]. The proportion of patients with ECOG performance status 0 varied from 60–62% in five studies [Citation26,Citation27,Citation30–32], compared to 50–55% in the remaining three studies [Citation25,Citation28,Citation29]. The frequency of patients with visceral metastases varied from 49% [Citation28,Citation29] to 56–60% [Citation26,Citation27,Citation30,Citation31]. On the contrary, the rate of only bone metastases varied from 18% [Citation28] and 22% [Citation27,Citation29,Citation32] up to 27% [Citation25,Citation31]. The lowest median age of 57 years was seen in the PALOMA-3 study [Citation30], compared to the average median age in all the studies of 62 years. The primary endpoint was PFS in seven studies [Citation26–32] and time to progression (TTP) in one [Citation25].
Table 1. Study information and patient populations.
Limitations within the trials
Limitations relevant for the assessment of the primary outcome for efficacy (PFS/TTP) in the eight RCTs included in this review are also listed in Table A in supplementary material. All eight RCTs report the outcomes of the intention to treat (ITT) population. PALOMA-3 and MONARCH 2 are adequately randomized and double-blinded trials, where the results including the PFS and clinical benefit rate (CBR) are analyzed by a central independent review committee, and therefore do not have any risk of serious bias [Citation30,Citation31]. MONALEESA-2 is also adequately randomized and double-blinded. However, the PFS described in the study was based on local assessment, and the only result listed from the central review was a HR, causing a risk of detection bias of the PFS [Citation27]. MONALEESA-2 was stopped at the interim analysis [Citation27], and stopping a trial prematurely due to potential benefit might overestimate the treatment effects [Citation23]. PALOMA-2 and MONARCH 3 have an increased risk of selection bias, due to an imbalance in the distribution of patients in performance status 0 and in the treatment-free interval ≥36 months, respectively, favoring the intervention groups [Citation29,Citation32]. Bachelot et al. and PALOMA-1 are open label trials, with some imbalance in the patient characteristics in favor of the intervention groups [Citation25,Citation28]. Furthermore, the outcomes were only investigated by local reviewers, and there is thus a risk of selection, performance and detection bias. There were few patients lost to follow-up in all studies combined, and the rest of the attritions were accounted for. Information about allocation concealment was unclear in all eight studies, however blinded trials are very likely to be concealed, and thus only the two open-label trials have a risk of bias [Citation23]. There were differences in the distribution of attritions in two of the trials, which present a risk of attrition bias and hereby a risk of selection bias; in BOLERO-2, 8.5% of the intervention group versus 2.5% of the ET only group left the trial early [Citation26], and in PALOMA-1, the attrition was 7.1% versus 13.6% [Citation28]. In the two trials where final overall survival data were available, crossover was not allowed [Citation25,Citation28]. In total, the majority (five) of the trials has no serious limitations (Table A in supplementary material), and consequently the quality of evidence is not downgraded for risk of bias [Citation23].
Efficacy
The efficacy results reported in the eight RCTs are listed in . In terms of first-line trials, the two palbociclib trials reported a median PFS of 20.2 months in the combination group versus 10.2 months in the ET only group (the corresponding hazard ratio (HR) for disease progression or death was 0.49; 95% CI 0.32–0.75; one-sided p < .0001) [Citation28], and 30.5 versus 19.3 months (HR 0.65; 95% CI 0.51–0.84; p = .001) [Citation29]. It suggests an increase of the PFS of 10–11 months when adding palbociclib to ET. The PFSs were not reached in the first-line abemaciclib trial (HR 0.51 (0.36–0.72; p = .0001) [Citation32], nor in the ribociclib group in MONALEESA-2, where the HR determined by blinded reviewers was 0.59 (95% CI 0.43–0.72; p = .002) [Citation27], both suggesting a significant benefit from adding a CDK4/6 inhibitor. Similarly, the trials assessing the response in patients previously treated for metastatic disease show a significantly increased PFS of at least five to six months. The PFS in PALOMA-3 was 9.5 months in the palbociclib arm vs 4.6 months in the placebo arm (HR 0.46; 95% CI 0.36–0.59; p < 0,0001) [Citation30], and MONARCH 2 found a PFS of 22.4 in the abemaciclib arm versus 10.2 months in the placebo arm (HR 0.46; 95% CI 0.36–0.58; p < .001) [Citation31]. The everolimus trials reported a PFS of 10.6 versus 4.1 months in the placebo group (HR 0.36; 95% CI 0.27–0.47; p < .001) [Citation26], and a median TTP of 8.6 versus 4.5 months in the ET only group (HR: 0.54; 95% CI 0.36–81; p = .0021) [Citation25]. The objective response rate (ORR) of the ITT population was as expected highest in the first-line trials. Five studies presented a significant increase of the ORR [Citation26,Citation27,Citation30–32], and six trials presented a significant increase of the clinical benefit rate (CBR) [Citation25,Citation27–31]. Median overall survival (OS) was only reported in the two randomized phase II trials, of which one was not statistically significant [Citation28]. In Bachelot et al., the median OS was not reached in the everolimus group and was 32.9 months in the ET only group, yielding a significant HR at 0.45 (95% CI 0.24–0.81) [Citation25]. However, the final OS analysis of the BOLERO-2 trial did not show a statistically significant improvement of the OS (31.0 versus 26.6 months; HR= 0.89; 95% CI 0.73–1.10; p = .14) [Citation33].
Table 2. Efficacy outcomes of included clinical trials.
Adverse events
The mTOR inhibitor everolimus
In Bachelot et al. [Citation25] and BOLERO-2 [Citation26] the most common grade 3 and 4 adverse events (AEs) in the everolimus groups included stomatitis (8% and 11%), anemia (6% and 2%), pneumonitis (3% and 2%) and hyperglycemia (4%). These adverse events (AEs) only occurred in 0–1% of the ET only group. Other common AEs of any grades in the everolimus arms were: fatigue, rash, anorexia and diarrhea. In BOLERO-2, serious AEs occurred in 23% of patients in the everolimus group and in only 12% in the ET only group [Citation26]. In total, 19% discontinued everolimus treatment because of AEs (versus 4% in the placebo arm) in the BOLERO-2 study [Citation26], and 11% (versus 4%) in the study by Bachelot et al. [Citation25]. The death of 1.4% of patients was considered to be attributable to AEs caused by everolimus [Citation26]. No deaths were reported by Bachelot et al. [Citation25].
The CDK 4/6 inhibitors ribociclib, palbociclib and abemaciclib
The most common grade 3 and 4 AE of the CDK 4/6 inhibitors was neutropenia. The rates were highest in the ribociclib-; 59.3% [Citation27] and palbociclib trials; 54%, 66.4% and 65% [Citation28–30], compared to 26.5% and 21.1% in the abemaciclib trials [Citation31,Citation32]. The corresponding rates in all placebo groups were 1–2%. Other common grade 3 and 4 AEs were leukopenia (19%, 24.8% and 28%) and anemia (6%, 5.4% and 3%) in the palbociclib groups [Citation28–30]; diarrhea (13.4% and 9.5%), leukopenia (8.8% and 7.6%), anemia (7.2% and 5.8%) and elevated alanine aminotransferase (ALT) level (4.1% and 6.1%) in the abemaciclib groups [Citation31,Citation32]; and for the ribociclib group: leukopenia (21%), lymphopenia (6.9%) and increased ALT- (9.3%) and aspartate aminotransferase (AST) level (5.7%) [Citation27]. In all trials, other common lower grade AEs included hematological toxicities (neutropenia, leukopenia, anemia), fatigue, infections, diarrhea, nausea, abdominal pain, arthralgia and alopecia. In the abemaciclib arms, 86.4% and 81.3% experienced diarrhea of any grade [Citation31,Citation32]. Serious AEs occurred in 21.3% (vs 11.8% in the placebo arm) in the ribociclib trial [Citation27]; in 19.6% and 13% (versus 12.6% and 17%) in the palbociclib trials [Citation29,Citation30]; and in 22.4% and 27.5% (vs 10.8% and 14.9%) in the two abemaciclib trials [Citation31,Citation32]. Discontinuation of treatment due to AEs occurred in 7.5% (versus 2.1% in the placebo arm) of patients in the ribociclib study [Citation27]; in 13%, 9.7% and 4% (versus 2%, 5.9% and 2%, respectively) in the palbociclib studies [Citation28–30]; and in 15.2% and 19.6% (vs 3.1% and 2.5%) in the abemaciclib trials [Citation31,Citation32]. AEs led to the death of 2.4% and 2.0% of patients in the abemaciclib arms (vs 1.2% and 0.9% in the placebo arms) in MONARCH 2 and -3, respectively [Citation31,Citation32]. No deaths were directly linked to the toxic effect of palbociclib in any of the three trials [Citation28–30]. In the ribociclib group, 2.7% experienced QTcF prolongation, leading to one death (among 334 patients) [Citation27].
Discussion
Available randomized studies of the mTOR inhibitor everolimus (two trials) and the CDK 4/6 inhibitors ribociclib, palbociclib and abemaciclib (six trials) in addition to standard endocrine therapy in women with HR+/HER2− MBC all showed a significant increase in PFS/TTP, compared to monotherapy with ET. These findings were evident across different study populations, both as first-line treatment and among patients previously treated for metastatic disease, suggesting a clinically meaningful delay in the development of endocrine resistance.
This review is based on a systematic literature search, that yielded all available studies of the four targeted agents in addition to endocrine therapy in women with HR+/HER2− MBC. The included studies were all systematically evaluated using well-defined criteria from the GRADE approach to rate the quality of evidence [Citation23]. In this review, the GRADE approach was slightly adapted and further elements were included to obtain a broader evaluation. Most information is from studies with low risk of bias, and according to the GRADE guidelines, the true effect of the PFS will be close to the estimated effect of the PFS [Citation23]. In three studies, there were a high risk of bias, and it can be argued only to focus on the highest quality trials. However, the effect sizes were similar in all studies and precision would be lost if these studies where omitted [Citation23]. Furthermore, the number of published studies is still limited. One limitation of systematic reviews is the potential risk of publication bias. Additionally, the systematic review is based on study results and not at an individual level as in higher quality meta-analyses. The primary endpoints in all trials were PFS or TTP, and the clinically most important endpoint, overall survival, is still awaited in most of the trials. Since the overall survival results can be difficult to obtain due to long follow-up and potential confounding from post study therapies, an ASCO working group has acknowledged PFS as a secondary clinically meaningful endpoint, as symptoms caused by cancer progression are important to patients’ quality of life [Citation34].
The eight trials present improvements of the PFS/TTP similar to the effect sizes previously seen in the aromatase inhibitor (AI) vs tamoxifen trials [Citation7,Citation8], that have led to the preference of AIs as first-line therapy in postmenopausal women with HR+/HER2− MBC [Citation35], and the results have already led to the approval of all four targeted agents. Everolimus, palbociclib and ribociclib have all been approved by the FDA and EMA and are commercially available; palbociclib as first-line (in combination with an AI) and second-line (combined with fulvestrant), ribociclib as first-line (in combination with AI) and everolimus (in combination with exemestane) after progression on an AI. By November 2017, abemaciclib was approved by the FDA (in combination with fulvestrant) for patients with disease progression on ET.
All studies performed subgroup analyses according to baseline characteristics and additionally, PALOMA 3 included PIK3CA mutation status and level of ER expression in the analysis. In all trials, the TTP/PFS results were consistent across all subgroups and so far, there is no data suggesting which patient groups will benefit the most when treated with CDK 4/6 inhibitors or the mTOR inhibitor.
The effect sizes across studies are similar for everolimus, ribociclib, palbociclib and abemaciclib. The CDK 4/6 inhibitors were generally well tolerated, as the most common AEs were due to myelosuppression, whereas the mTOR inhibitor caused more severe AEs with stomatitis being the most frequent, raising an argument for the preference of CDK 4/6 inhibitors rather than the mTOR inhibitor. Currently, no data on potential use of a different CDK4/6 inhibitor or everolimus after progression on a treatment including one CDK4/6 inhibitor or everolimus is available. Based on efficacy only, there is no indication of preferring any of the three CDK 4/6 inhibitors in first- and second-line treatment. The differences in AEs across agents could help the clinician select among the CDK 4/6 inhibitors; e.g., for patients with heart disease ribociclib could be deselected due to the risk of QTcF prolongation and for patients suffering from colitis, deselecting abemaciclib might be preferred due to the high frequency of diarrhea. Another difference among the targeted agents is in administration, as abemaciclib is being administered twice daily compared to once daily for everolimus, palbociclib and ribociclib; administration multiple times daily is generally associated with a lower compliance [Citation36,Citation37]. Furthermore, as all new cancer therapies are expensive, and health care resources are limited in most countries, hence price could become a substantial argument for the choice of drug, due to the comparable efficacy.
Conclusions
The four new targeted agents are all associated with an improvement of the PFS and have an acceptable tolerability. Thus, they should be offered to women with advanced HR+/HER2− breast cancer both as first-line therapy as well as among patients previously treated for metastatic disease. However, further data regarding the impact on overall survival are required to evaluate the full benefit. As the effect is comparable, price and differences in AEs could become substantial arguments for the individual choice of therapy.
Supplemental Material
Download MS Word (32.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Perez EA. Treatment strategies for advanced hormone receptor-positive and human epidermal growth factor 2-negative breast cancer: the role of treatment order. Drug Resist Updat. 2016;24:13–22.
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)dagger. Ann Oncol. 2014;25:1871–1888.
- Kurebayashi J. Endocrine-resistant breast cancer: underlying mechanisms and strategies for overcoming resistance. Breast Cancer. 2003;10:112–119.
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247.
- Bonneterre J, Thürlimann B, Robertson JFR, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the tamoxifen or arimidex randomized group efficacy and tolerability study. J Clin Oncol. 2000;18:3748–3757.
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol. 2000;18:3758–3767.
- Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–4890.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the international letrozole breast cancer group. J Clin Oncol. 2003;21:2101–2109.
- Iwata H, Masuda N, Ohno S, et al. A randomized, double-blind, controlled study of exemestane versus anastrozole for the first-line treatment of postmenopausal Japanese women with hormone-receptor-positive advanced breast cancer. Breast Cancer Res Treat. 2013;139:441–451.
- Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005.
- Tryfonidis K, Zardavas D, Katzenellenbogen BS, et al. Endocrine treatment in breast cancer: cure, resistance and beyond. Cancer Treat Rev. 2016;50:68–81.
- Provenzano A, Kurian S, Abraham J. Overcoming endocrine resistance in breast cancer: role of the PI3K and the mTOR pathways. Expert Rev Anticancer Ther. 2013;13:143–147.
- Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413.
- Yue W, Fan P, Wang J, et al. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–110.
- Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–5328.
- Rocca A, Schirone A, Maltoni R, et al. Progress with palbociclib in breast cancer: latest evidence and clinical considerations. Ther Adv Med Oncol. 2017;9:83–105.
- Vidula N, Rugo HS. Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin Breast Cancer. 2016;16:8–17.
- Butt AJ, McNeil CM, Musgrove EA, et al. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Relat Cancer. 2005;12: S47–S59.
- Spring L, Bardia A, Modi S. Targeting the cyclin d-cyclin-dependent kinase (CDK) 4/6-retinoblastoma pathway with selective CDK 4/6 inhibitors in hormone receptor-positive breast cancer: rationale, current status, and future directions. Discov Med. 2016;21:65–74.
- Alves CL, Elias D, Lyng M, et al. High CDK6 protects cells from fulvestrant-mediated apoptosis and is a predictor of resistance to fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016;22:5514–5526.
- Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–837.
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77.
- Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence-study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394.
- Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–2724.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748.
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35.
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936.
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439.
- Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884.
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646.
- Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol. 2014;25:2357–2362.
- Ellis LM, Bernstein DS, Voest EE, et al. American Society of Clinical Oncology Perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol. 2014;32:1277–1280.
- Cardoso F, Harbeck N, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii11–vii19.
- Falagas ME, Karagiannis AK, Nakouti T, et al. Compliance with once-daily versus twice or thrice-daily administration of antibiotic regimens: a meta-analysis of randomized controlled trials. PLoS One. 2015;10:e0116207.
- Laliberte F, Bookhart BK, Nelson WW, et al. Impact of once-daily versus twice-daily dosing frequency on adherence to chronic medications among patients with venous thromboembolism. Patient. 2013;6:213–224.
