Abstract
Objective: This prospective pilot study assessed the feasibility of electronic email-based questionnaires about patient-reported complications after colonoscopy.
Material and methods: A newly internally validated questionnaire on patient-reported complications related to colonoscopy was conducted as an online survey.
Results: Out of 200 patients (mean age 65 years), 83% completed the first questionnaire immediately after the procedure, 77% completed the second follow-up questionnaire after 24 h at home, and 70% the third one after 30 d. Forty-four percent of the patients reported minor adverse events after 24 h, and 23% at the follow-up after 30 d. The rate of sick leave in the 30-d period after the colonoscopy was 6%.
Conclusions: This study shows that email-based questionnaires give a high response rate independent of age or gender, but that the response rate declines with time after colonoscopy. Minor adverse events are underestimated, and colonoscopy could lead to sick leave in a minor subgroup of patients.
Introduction
After the introduction of population-based screening for colorectal cancer in Denmark in 2014, almost 20,000 colonoscopies are performed per 1m citizens. This number will increase further due to the screening program [Citation1]. According to the Danish Colorectal Cancer Screening Database, the frequency of serious complications from colonoscopy is 0.23% in Denmark. A retrospective survey based on a systematic review of 246 patient files from the screening database revealed a complication rate of 0.71%, of which approximately 45% were mild [Citation2]. A systematic review from the Netherlands found a mortality rate of 2.9/100,000 colonoscopies. Major complications after colonoscopy were perforation/laceration, bleeding, infection and post-polypectomy syndrome [Citation3].
Little is known about minor adverse events after colonoscopy, because the patients leave the clinic after the procedure and have renewed contact with the hospital only in case of severe complications. The actual costs for society of a colonoscopy are largely unknown and the rate of sick leaves contributes as an important factor. According to bowel screening figures from Denmark, the reporting of adverse outcomes related to colonoscopy indicates a severe underreporting of major complications as well [Citation4].
This is probably due to the fact that some patients are readmitted to another medical facility than the one performing the colonoscopy. There is a need for patient-based reports to quantify adverse events outcome and test if it is possible to collect reports based upon email contact. However, the population of interest has a high mean age and their compliance with email-based reporting is unknown.
Therefore, the objective of this prospective study was to test the feasibility of using email links to a database containing questionnaires on patient-reported minor adverse events related to colonoscopy performed on an outpatient basis. Secondary objectives were to enable a further refinement of the questionnaires and to preliminarily investigate the frequency of minor adverse events and sick leaves after colonoscopy in our population.
Material and methods
Patient selection
The enrollment was carried out over three weeks in February 2017 at an endoscopy unit in Nyborg, Odense University Hospital, Denmark. The clinic performs 9000 colonoscopies yearly.
The inclusion criteria were adult age (over 18 years old) and admission for colonoscopy. Patients were excluded if the colonoscopy was incomplete (not reaching the cecum or the ileocolic junction or incomplete cleansing), newly diagnosed cancer at colonoscopy, if the patients were unwilling to comply or unable to understand Danish. Also, patients who were mentally or cognitively unable to participate were excluded.
The indications for colonoscopy included abdominal pain, diarrhea, changes in bowel habits, blood in feces, weight loss, anemia, family history of colorectal cancer, positive hemoglobin stool test in the screening program or surveillance colonoscopies. All referrals from general practitioners were accepted for colonoscopy without censorship.
Ethics
The study followed the Declaration of Helsinki on medical protocol ethics and was approved by the Danish Data Protection Agency (Journal no. 16/17609). All patients gave informed consent and the study was approved by the Danish National Committee on Health Research Ethics (Journal no. 2012-58-0018). The investigators considered it unethical to include patients with newly diagnosed or suspected cancer at the colonoscopy. It seemed inappropriate to ask these patients about minor adverse events. Also, the probability for preexisting symptoms as well as symptoms after the colonoscopy was considered greater in patients with a tumor, giving a risk of biased data.
Methods
The questionnaires were developed on the basis of side effects reported in the national screening database, the literature, and the investigators’ clinical experience. Afterward, the questionnaires were tested for validity, comprehensibility and reliability by our patient advisory board. In this particular study, the patient advisory board participated in the selection of complications to be addressed based on their own experiences undergoing a colonoscopy. Furthermore, they approved the questionnaires in terms of relevance and comprehensibility of the questions. The questionnaires were revised accordingly. Reliability testing of the study was performed by exposing the patients advisory board to the questionnaires, the degree to which the results obtained by the answering of the questionnaires procedure could be replicated from the Electronic Patient-Reported Outcome Measures (ePROM) was thought to be sufficient by the investigators and the patient advisory board. The validity was tested in a similar manner by exposing the questionnaires to expert endoscopist being the investigators, and the patient advisory board who all went through a colonoscopy and could thereby be regarded as empiric experts.
The final questionnaires were integrated into a secured database: ‘Odense Patient Data Explorative Network (OPEN®)’ and the ‘Research Electronic Data Capture (REDCap®)’. REDCap® is a secure, web-based application designed to support data capture and analysis for research studies. The patients were presented with a questionnaire in Danish language. The questionnaire was later back-translated to English language for the paper by the authors.
Patients gave an email address for receiving the questionnaires before the procedure started. Afterward, the email addresses of the participants were registered along with demographic baseline information and were filed in REDCap® by the nurse. The email addresses given were not verified. The patients then received a baseline questionnaire, which they completed at the clinic.
New questionnaires were automatically generated and sent 24 h and 30 d after the index colonoscopy, respectively. The patients completed these in their own homes. For a questionnaire to be completed and sent the patients had to answer all questions. The questionnaires for after 24 h and 30 d were not identical. Patients had the opportunity to report other complications than those listed in the questionnaires by using the available text boxes. If the patients did not reply within 24 h, they would receive one reminder by email. All information from the questionnaires was automatically saved in the database. For a complete list of questions, see Supplementary Appendix 1.
Minor adverse events were defined as any health problem experienced during the 30-d period after the procedure. If patients were readmitted to the hospital after discharge, or were not discharged on the day of the procedure, the events were classified as major. As the patients’ experiences were the aim of the study, a visit to the general practitioner caused by symptoms also was defined as a major adverse event. If patients answered that they were readmitted to the hospital, the medical record would be checked for diagnosis during admission. The patients could report both major and minor adverse events. Concerning socioeconomic factors, it was chosen to focus on sick leaves in this study.
Colonoscopy/procedure method
Patients were given PicoPrep® (citric acid, magnesium oxide and sodium picosulfate) as bowel preparation the day before colonoscopy. All colonoscopies were performed using carbon dioxide for insufflation. Patients were sedated with Pethidine and Midazolam if needed. Bowel cleanliness was described according to a four-category scale (Boston Bowel Preparation Scale (BBPS)) [Citation5].
Statistical analysis
Summary statistics were calculated as appropriate (means and standard deviations/median and interquartile range for continuous variables, numbers and proportions for categorical variables). The descriptive analyses were performed using STATA version 14 (StataCorp, College Station, TX, USA). An independent t-test was used to test differences between the different outcome measures. p > .05 to indicate statistically significant different results. The t-test was chosen when the assumption of a normal distribution was achieved for the outcome measures of interest. A Wilcoxon rank-sum analysis was used if normal distribution was not achieved.
Results
Participants
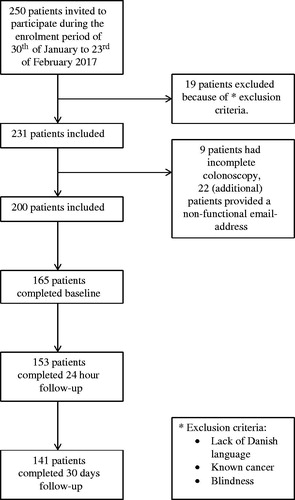
Out of 250 consecutive patients, 19 (8%) were not included according to predetermined exclusion criteria such as lack of Danish language or known cancer and one of them was excluded because of blindness. Blindness was not a predetermined criterion, but necessary for practical reasons. Of the 231 patients eligible for participation, 9 patients (4%) had incomplete colonoscopies, and 22 (10%) were not included because the email addresses entered were incorrect (). The 200 included patients had the median age of 65 years (32–88) and 103/200 (51%) participants were male ( and Citation2).
Table 1. Baseline questionnaire (patients).
Table 2. Colonoscopy characteristics (Nurse baseline questionnaire).
Survey responders vs. non-responders
The response rate after 1 h, concerning patient baseline, was 83% (165/200). At the 24-h follow-up, 77% responded (154/200) and after 30 d 70% responded (140/200). There was no statistical difference (p=.89) in age or gender between responders and non-responders.
Baseline questionnaire
Characteristics of included patients
The most common reasons for referral to colonoscopy were blood in stool 61/200 (31%), abdominal pain 45/200 (23%), and changes in stool habits 39/200 (20%). Other reasons were participation in the national bowel cancer screening program 29/200 (15%), surveillance after polypectomy 25/200 (13%), family history of colorectal cancer 12/200 (6%), weight loss 10/200 (5%) and anemia 5/200 (2.5%).
The patients were asked how much they feared the colonoscopy before the procedure on a scale from 1 to 100, which resulted in a mean score of 44 (± 29). Ninety percent of the participants reported their bowel cleansing to be complete after ingestion of PicoPrep® in preparation for the colonoscopy. Almost half of the patients (45%) experienced nausea during bowel cleansing, and 11% vomited. Concerning pain during the colonoscopy, the patients reported a mean of 38 (± 29) on a scale from 1 to 100.
Colonoscopy baseline characteristics
The mean duration of the colonoscopies was 22 min. The nurses reported that 128/200 (64%) of the patients presented with good bowel cleanliness. All included patients had acceptable bowel cleanliness or better.
A total of 80% of patients received Pethidine and the mean dose was 18 (±10) mg. Also, 81% were sedated with Midazolam sedation with a mean dose of 2 (±3) mg. For all baseline characteristics, see and Citation2.
24 hours questionnaire
The most common minor adverse events 24 h after colonoscopy were abdominal discomfort 39/154 (25%), headache 27/154 (17%), bloating 24/154 (15%) and abdominal pain 21/154 (14%). Other complications were nausea 9/154 (6%), fever 3/154 (2%), rash 2/154 (1%) and emesis 1/154 (1%). One patient (1%) reported dizziness and 4/154 (3%) reported flatulence. Almost half of the participants 67/154 (44%) reported some form of complication/side effect after 24 h. There was no statistical difference (p = .217) in age (over/under 64.5 years) for the amount of minor adverse events reported. When looking at patients who did not report any minor adverse events, 52/79 (66%) were over 64.5 years and 35/75 (47%) were younger (p = .001). Women reported significantly fewer complications than men (p = .004).
30 days questionnaire
Minor adverse events
The 30 d questionnaire was completed by 140 (70%) patients. Six patients (4%) still experienced pain from the colonoscopy. Nineteen (14%) were still bloated, but 14 of these patients were bloated before the procedure as well. Fifteen patients (11%) reported that their stool had changed after the colonoscopy, mostly to become more fluid. In addition to the minor adverse events listed in the questionnaire, six (4%) patients reported the following: pain due to a painful polyp, bloating, abdominal pain from bloating and dizziness. The total number of patients with minor adverse events (not present before the procedure) at the 30 d questionnaire was 32 (23%). A total of eight (6%) patients reported a worrisome bleeding from the bowel at some point between the colonoscopy and follow-up after 30 d.
Major complications
Ten patients were classified with a major complication.
Seven (5%) patients consulted their general practitioner because of symptoms caused by the colonoscopy. Three (2%) patients reported that the colonoscopy leads to hospitalization for one day. No mortality was observed in this cohort.
Sick leaves
Eight patients (6%) had a sick leave from work because of the colonoscopy. One patient was on sick leave for one day, three patients for three days, one for four days and one for five days. However, there were two patients who needed a sick leave for more than one week. The sick leaves were consecutive days after the colonoscopy.
For a complete list of results see .
Table 3. Results: total number of minor adverse events after 24 h follow-up.
Table 4. Results: statistics for minor adverse events after 24 h follow-up.
Table 5. Results: type and amount of minor adverse events after 24 h follow-up.
Table 6. Results: 30 d follow-up questionnaire.
Discussion
Obtaining data through email is feasible with an acceptable response rate between 83% and 70%. The response rate is low after 30 d, but it is probably not relevant to obtain data at this time and this might in part explain the low participation rate. A third questionnaire after 14 d is being considered as an alternative. The text boxes in the questionnaires were used very rarely and the questionnaires will be used almost unchanged for further studies. The participation rate could perhaps be increased by introducing a procedure to ensure the correctness of the registered email addresses, as 10% of non-responders were caused by wrong email addresses. A further increase in completion rates could be obtained if the questionnaires would permit to leave some of the questions unanswered, but this might lead to more incomplete answers. In this study, it was decided not to do statistical calculations in relations to response rates and decline with time after the procedure, as the reply rate was expected to decline. There was no statistical difference (p=.89) in age or sex between the responders and the non-responders. However, in this feasibility study it cannot be excluded that there are some socio-demographic differences between responders and non-responders. The most obvious possible bias expected was a lower response rate in high age because of limited email experience in this group, but this was not the case.
This study found that women report significantly fewer complications than men. This finding is in contrast to the studies from the Netherlands [Citation6] and Taiwan [Citation7], which found that women report more complications than men.
At the 24 h questionnaire, 3% of patients reported flatulence, which was not listed in the questionnaire. It might be that the investigators think of air in the digestive tract as bloating, whereas some patients describe the complication as flatulence. The reported cases of headache could be due to PicoPrep® used before the colonoscopy, since this side effect is common (1–10%). Also, the percentage of patients vomiting during the cleansing procedure is relatively high (11%).
There were some limitations to this study. The endoscopists had different levels of experience, which also could have had an impact on the results. Both patients with diagnostic and therapeutic (biopsies) colonoscopies were included, which could cause different exposures causing abdominal pain. No resectional procedures were conducted in this study. The study had a small sample size and a single-center setting because it was intended as a feasibility study to evaluate the questionnaires.
The study found a high rate of complications (44%) during the first 24 h. The real incidence of adverse events is underestimated both in number and in impact when only major adverse events are counted [Citation6]. Therefore, the true outcome lies in each parameter enquired, e.g., bloating, abdominal pain, abdominal discomfort, nausea, constipation, etc.
We tried to identify the true side effects by asking patients if their symptoms had also been present before the procedure. However, when asking patients about complications at follow-up after 30 d, there is a risk of bias because the symptom might not originate from the colonoscopy but something else that had evolved in the meantime.
It is important to address minor adverse events that do not result in hospitalization but cause significant discomfort for the patient and this is a core issue in quality control for colonoscopy [Citation6,Citation8]. Marquez et al. reported that 4/420 (1%) patients were in need of medical consultations after the colonoscopy.
In this study, 95% of the patients returned to their daily activities before 30 d follow-up. However, 6% of the patients had excused absence from work because of the colonoscopy. Sick leaves were not tested statistically by comparing to a control group, so it is uncertain if this 6% is significant or not. There is also a possibility that the reason for the sick leave was other than the colonoscopy. For future studies, the control group could be asking the patients if they had any sick leaves in the last three months before the colonoscopy.
To be allowed to contact patients after the procedure, they had to confirm this at the baseline questionnaire. The total percent of patients lost to follow-up was 30%. Since 18% of the 200 patients were lost to follow up at baseline, it did not seem relevant to check sick leaves among the remaining 12% non-responders at 24 h and 30 d follow up. However, this could be solved in a future study by having the patients to consent to be contacted when they provide an email address to the nurse.
Marquez et al. [Citation8] collected answers by email and an internet-based survey. When participants were not reached, they were telephoned daily for the next three consecutive days. However, they still had the lowest response rate (64%) amongst comparable studies [Citation8]. Lee et al. had a 100% response rate at their end-point of 24 h follow-up, but it is quite unclear how this was obtained [Citation7]. Ko et al. and de Jonge et al. also had high response rates by telephone of 94% and 75%, respectively [Citation6,Citation9].
This study is the first to conduct an automated internet-based follow-up on minor adverse events after colonoscopy. Previous studies have been conducted between 2006 and 2014, where online surveys might not have been so common. However, now in 2018, it is easy and time-saving to conduct an internet-based follow-up. This enables studies on large populations.
Conclusion
This prospective study shows that online surveys give acceptable response rates for patients who undergo colonoscopy during a limited time after the procedure. The time has come where also older citizens can manage email questionnaires, and this design for patient-reported outcome seems promising. This study shows that no statistical difference existed in age or sex between responders and non-responders. Our results indicate that minor adverse events after colonoscopy are still underestimated. A future study should include a larger group of participants to increase the statistical power of a more detailed subgroup analysis. A checking system for correct email addresses should be implemented. The following demographic data should be present; age, gender, reason for referral, use of blood thinners, type (therapeutic and diagnostic) and description (use of sedatives, duration, cleanliness and completion) of procedure. It could also be interesting to include the incomplete colonoscopies as a separate group to see if they experience more adverse events. When reporting minor adverse events, preexisting symptoms should be differenced. All remittances to the hospital should be controlled with a diagnosis code.
Supplemental Material
Download MS Word (16.2 KB)Acknowledgments
Odense Patient Data Explorative Network (OPEN) is thanked for their assistance in designing the email-based questionnaire system and electronic database.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sundhedsstyrelsen, “Tarmkraeft-screening,” 2016. Available from: https://sundhedsstyrelsen.dk/da/sygdom-og-behandling/screening/ tarmkraeftscreening#
- “Dansk tarmkraeftscreeningsdatabase Årsrapport.” 2014. Available from: https://www.sundhed.dk/content/cms/45/61245_dtsårsrapport-2014_8-1-16_final_inklbilag.pdf
- Reumkens A, Rondagh EJA, Bakker CM, et al. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016;111:1092–1101.
- “The Danish Clinical Registries (RKKP), Dansk Tarmkraeftscreening Database.” 2014. Available from: http://www.rkkp.dk/in-english/
- Calderwood AH, Jacobson BC. Comprehensive validation of the Boston bowel preparation scale. Gastrointest Endosc. 2010;72:686–692.
- de Jonge V, Nicolaas JS, van Baalen O, et al. The incidence of 30 day adverse events after colonoscopy among outpatients in the Netherlands. Am J Gastroenterol. 2012;107:878–884.
- Lee YC, Wang HP, Chiu HM, et al. Factors determining post-colonoscopy abdominal pain: prospective study of screening colonoscopy in 1000 subjects. J Gastroenterol Hepatol. 2006;21:1575–1580.
- Marquez Azalgara V, Sewitch MJ, Joseph L, et al. Rates of minor adverse events and health resource utilization postcolonoscopy. Can J Gastroenterol Hepatol. 2014;28:595–599.
- Ko CW, Riffle S, Shapiro JA, et al. Incidence of minor complications and time lost from normal activities after screening or surveillance colonoscopy. Gastrointest Endosc. 2007;65:648–656.