Introduction
Historically, academic medical centers conducted oncologic clinical research trials, which limited trial access for the majority of cancer patients, who are treated at community centers. Over the last several decades, national groups in the United States have pursued efforts to improve the quality of cancer care in the community as well as increase clinical trial capabilities at these community centers; these efforts include the National Cancer Institute (NCI) Community Clinical Oncology Program (CCOP), the Minority-Based CCOP and the NCI Community Cancer Centers Program (NCCCP) [Citation1–3]. Despite these efforts, <5% of adult American cancer patients enroll on clinical trials, although 70% of Americans would be ‘inclined or very willing’ to participate in such trials [Citation4,Citation5]. Many barriers exist to clinical trial accrual, including structural and clinical barriers, which may be particularly relevant in community practices [Citation5].
The University of Texas MD Anderson Cancer Center (MDACC) has 4 community satellite centers known as Houston-Area Locations (HALs), all within 35 miles of the main MDACC campus in the Texas Medical Center in Houston. These 4 community satellite centers offer multidisciplinary clinics across a range of oncologic disease sites, and include faculty specializing in radiation oncology, surgical oncology, medical oncology and gynecologic oncology, among others. The radiation oncology service was the first satellite service to be offered at the HALs, beginning in 2004. Typically, patients seen at the HALs who desired clinical trial access were screened and enrolled by the attending physician on service at the HAL. With continued growth of these satellites, to a total of 10 HAL radiation oncology faculty members, the institution formed a dedicated research team to facilitate trial design and enrollment by the HALs. Yet, even with these research personnel, accrual to clinical trials remained low, with only 3% of patients seen in consultation with a HAL radiation oncologist enrolling to a clinical trial in fiscal year 2016 (FY16, September 2015–August 2016). In order to improve clinical trial enrollment among HAL radiation oncology faculty, we implemented a quality improvement project utilizing the Plan-Do-Study-Act model [Citation6]. Combining a monthly clinical trials scorecard with monthly research-oriented conference calls, these efforts markedly improved clinical trial enrollment as well as screening by over three-fold.
Material and methods
The 4 MDACC HAL satellite centers (known by their locations as Katy, Bay Area, Sugar Land, and Woodlands) include 10 staff radiation oncologists. These centers have robust multidisciplinary teams, including representation across oncologic disciplines, as well as a dedicated research team aimed at improving research participation at the HALs. All enrolling physicians at the HAL centers are trained on all eligible clinical trials available; site initiation visits are conducted for each HAL center prior to a protocol opening at the location, and all physicians complete both delegation of authority and training logs.
Toward the close of FY16, low rates of clinical trial accrual were noted among patients seen in consultation with a HAL radiation oncologist, consistent with the prior fiscal year. The research team reviewed barriers to enrollment as well as methods to overcome these barriers. Barriers to enrollment included (1) a large number of potential clinical trials, at varying stages of development and openness to enrollment; and (2) limited communication across teams at the 4 HALs to review trial-specific issues including enrollment criteria and insurance barriers to trial accrual.
To overcome these challenges, we implemented a two-part approach to improve screening and enrollment among HAL radiation oncology patients, including a monthly research-oriented conference call as well as a monthly trial scorecard. The monthly conference call, including both HAL and main campus radiation oncology physicians, focused on educating HAL physicians regarding currently-open protocols, as well as any issues affecting screening and/or enrollment on these protocols. Trial-specific issues discussed included concerns ranging from insurance approval delays affecting enrollment to specific eligibility criteria that may present challenges for HAL patients (i.e., – audiology, which necessitates a separate trip for patients to the main MDACC campus).
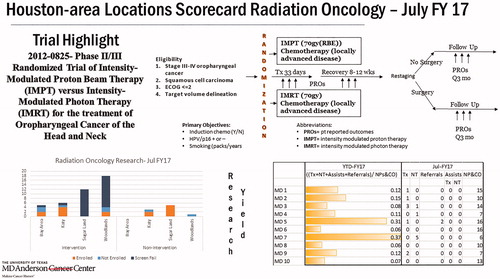
The monthly scorecard, distributed to all 10 HAL radiation oncologists as well as HAL clinical and research staff, included details of one particular clinical trial to increase awareness of trial eligibility criteria and design (). This monthly scorecard also provided information for the prior months’ enrollment data, at each of the 4 HALs and for each individual attending physician (). The scorecard itself was developed iteratively, following a retrospective review of HAL trial enrollment and screening data and through surveys of HAL staff. Notably, an identified major bottleneck to study accrual was a lack of motivation by attending physicians; the scorecards were designed to incentivize trial participation and enrollment. Furthermore, protocols were categorized by disease site specialty, and HAL attending physicians were assigned leadership roles to ‘champion’ each disease site specialty trials. The monthly scorecard allowed quantitative evaluation of our clinical trial portfolio, providing a quick and explanatory visual to demonstrate whether study objectives were met (). Additionally, real time data helped identify underperforming studies, which would be discussed at the monthly conference calls as above.
Figure 1. Sample scorecard. Sample scorecard provided as part of intervention to increase trial enrollment. Scorecard includes one open protocol in the top half of the card, providing key information on eligibility and intervention for a given protocol. Here, details are provided regarding internal institutional protocol 2012-0825, in which AJCC 7th edition Stage III–IV oropharyngeal carcinoma patients are randomized to definitive proton (IMPT) versus photon (IMRT) radiotherapy. In the bottom-left portion of the card, information is given regarding monthly rates of enrollment and screening of patients for protocols; split by trials with therapeutic intervention (‘Intervention’) and those without (‘Non-Intervention’), absolute counts are provided for patients at each of the 4 HALs (Bay Area, Katy, Sugar Land, and Woodlands) with regard to enrollment. This includes patients who were screened but failed to pass a screen for a protocol (dark blue, ‘Screen Fail’), those who passed a screen but were not enrolled (light blue, ‘Not Enrolled’), and those who both passed a screen and were enrolled on a protocol (orange, ‘Enrolled’). In the bottom-right portion of the card, information is provided for each of the 10 HAL radiation oncologists as regards year-to-date (YTD) and specific month (for this card, July FY17) enrollment. These 10 providers are de-identified (‘MD 1’, ‘MD 2’, etc.). This includes the rate of new consults (NPs & CO, new patients and consults) who are enrolled on a clinical trial (Tx + NT + Assists + Referrals, therapeutic trial enrollment, non-therapeutic trial enrollment, & referred for enrollment to trial only performed at the main campus).
This two-part effort was initiated in September 2016, at the start of FY17. Data were analyzed at the completion of FY17 (August 2017) for the prior fiscal years to assess the impact of the intervention on clinical trial screening and enrollment. Pearson’s Chi-squared tests were utilized for 2x2 contingency table analyses as part of all statistical tests employed in this study.
Results
The two-part intervention (monthly conference call and monthly scorecard) began at the start of FY17. Comparing data from FY16 and FY17, rates of radiation oncology patient enrollment onto clinical trials at the HALs increased by more than 3-fold, from 3% to 10%, p < .001 (). Similarly, rates of new patients being screened for clinical protocols at the HALs increased significantly, from 11% to 39%, p < .001. Moreover, the proportion of patients enrolled on protocols out of those screened for protocols did not change from FY16 to FY17, from 24% to 26% (p = .66), suggesting that the intervention did not result in more indiscriminate screening of patients, but rather reflected an improvement in rates of both appropriate screening and enrollment of potentially-eligible patients (). In an effort to ensure the validity of these results, we analyzed the number of open protocols in each year, and it was similar between FY16 and FY17 (38 vs. 41), as was the number of open therapeutic radiation oncology protocols (8 vs. 9). Between FY16 and FY17, increased enrollment of HAL patients on radiation oncology protocols did not appear to affect rates of non-radiation-oncology protocol screening (19% vs. 24%) or enrollment (12% vs. 9%) in the HALs. Additionally, the rate of clinical trial enrollment among radiation oncology patients was stable from FY14 to FY16 (2% in FY14, 2% in FY15 and 3% in FY16).
Table 1. Summary of results.
Discussion
Clinical trial screening and enrollment rates improved among the target physicians at satellite cancer center facilities after implementation of a two-part strategy consisting of monthly conference calls as well as informative scorecards. With one year of follow-up on this project, we observed significant gains in both screening and enrollment on protocols among radiation oncologists at MDACC HAL community satellites, with over 3-fold increases in both screening and enrollment. With consistent numbers of open trials between years, and no impact on non-radiation-oncology protocol screening or enrollment, we hypothesize that our intervention resulted in increased awareness and education regarding accruing protocols for HAL physicians. Through regular communication regarding these protocols, including HAL-specific challenges with regard to patient enrollment on protocols, the larger combined clinical and research team better facilitated patient exposure to these trial options.
Completing a cycle of PDSA for this project, we now are looking to better refine this intervention, and build on it across other disciplines within our institution. We are exploring data-driven approaches to further optimize patient identification for currently-open trials at the HAL campuses, with the aim of increasing appropriate screening rates for eligible patients. Looking to the future, other forums are being designed to improve communication between HAL physicians and principal investigators at the main campus. Ensuring continued involvement of HAL physicians in protocol design and trial development gives further voice to the community providers seeking to enroll patients on these protocols. Along the same lines, multidisciplinary discussions are ongoing to promote a similar two-part approach to increase trial enrollment among surgical oncology, gynecologic oncology and medical oncology providers in the HALs. For groups or departments with a large number of active trials, we recommend a targeted approach where this two-part intervention is tailored to the disease site(s) most relevant to the providers in the community based on the community patient population. We encourage other cancer centers with satellite networks to consider a similar approach to foster enrollment and research collaboration between a central campus and community affiliates [Citation3,Citation7]. Through these efforts we hope to offer more patients the opportunity to participate in clinical trials, and elevate the quality of cancer care for all.
Disclosure statement
All authors report no conflicts of interest or relevant financial disclosures related to this work.
References
- Warnecke RB, Johnson TP, Kaluzny AD, et al. The community clinical oncology program: its effect on clinical practice. Jt Comm J Qual Improv. 1995;21:336–339.
- Minasian LM, Carpenter WR, Weiner BJ, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116:4440–4449.
- Dimond EP, St. Germain D, Nacpil LM, et al. Creating a “culture of research” in a community hospital: strategies and tools from the National Cancer Institute Community Cancer Centers Program. Clin Trials. 2015;12:246–256.
- Comis RL, Miller JD, Aldige CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830.
- Unger JM, Cook E, Tai E, et al. Role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2017;35:185–198.
- Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290–298.
- Hirsch BR, Locke SC, Abernethy AP. Experience of the National Cancer Institute Community Cancer Centers Program on Community-Based Cancer Clinical Trials Activity. J Oncol Pract. 2016;12:e350–e358.
