Abstract
Background: Toxicity profiles play a crucial role in the choice between specific palliative chemotherapy regimens. To optimize the quality of life for cancer patients, patients should be adequately informed about potential toxicities before undergoing chemotherapy. Therefore, we constructed TOXviews, a novel graphical presentation and overview of toxicity profiles to improve information provision about adverse events. As an example, we analyzed first-line chemotherapy regimens for advanced esophagogastric cancer (AEGC).
Methods: We searched PubMed, EMBASE, CENTRAL, ASCO and ESMO for prospective phase II or III randomized controlled trials (RCTs) on palliative first-line systemic treatment for AEGC until February 2017. We extracted proportions of Common Terminology Criteria for Adverse Events grade 1–2 (mild) and 3–4 (severe) adverse events from each chemotherapy arm and pooled these by using single-arm meta-analysis. Toxicity profiles per chemotherapy regimen were visualized in bidirectional bar charts with pooled proportions plus 95% confidence intervals. For comparative analysis, chemotherapy regimens were grouped in singlets, doublets and triplets.
Results: We included 92 RCTs with a total of 16,963 patients. TOXviews for 3 fluoropyrimidine singlets, 5 cisplatin-containing doublets (C-doublets), 10 fluoropyrimidine non-cisplatin containing doublets (F-doublets), 4 anthracycline-containing triplets (A-triplets) and 5 taxane-containing triplets (T-triplets) were constructed. C-doublets, A-triplets and T-triplets all showed an increased incidence of grade 3–4 adverse events and clinically relevant grade 1–2 adverse events compared to F-doublets.
Conclusion: TOXview provides a new graphical presentation and overview of chemotherapy toxicities. TOXviews can be used to educate physicians about the incidences of AEs of systemic therapy and improve informed decision-making.
Introduction
In order to maintain the quality of life for patients with cancer, tolerability of cytotoxic treatment is crucial. This is especially true in the case of advanced disease when curation is not an option and treatment is aimed at improving survival while maintaining the quality of life. Especially when survival outcomes of certain treatment regimens are similar, differences in toxicity profiles between treatment regimens may guide treatment decisions.
Accurate communication with patients about adverse events that might occur in different treatment regimens is especially important to promote shared decision-making. In turn, a well-informed decision can result in a better quality of life [Citation1,Citation2]. Thus, comprehensive overviews of toxicity are urgently needed [Citation3,Citation4]. Several studies have attempted to construct a graphical presentation format (e.g., bar charts and stream plots) to aid information provision regarding toxicity profiles [Citation5]. However, the currently available graphical methods to present toxicities are hard to interpret by physicians because of the lack of relevant data, such as number of participants, trials and confidence intervals in these presentations [Citation6]. Furthermore, most illustrations of toxicity that are available represent the adverse events of a single study. To our knowledge, no pooled overview of adverse events profiles including multiple studies for chemotherapy is available yet.
In our research, we suggest a novel graphical approach of presenting toxicity of systemic treatment regimens: TOXview. As a clinical example, we studied first-line chemotherapy regimens for advanced esophagogastric adenocarcinoma (AEGC), one of the major causes of cancer-related mortality worldwide [Citation7–9]. Currently, there is no world-wide consensus about the optimal first-line palliative chemotherapy for AECG and several regimens with similar survival outcomes are available thus making expected adverse events highly relevant for treatment decision making [Citation10].
Methods
Literature search
We updated our previously performed search in the databases Pubmed, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) and the meeting abstracts from the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) for randomized controlled trials (RCTs) up to February 2017 [Citation10]. Medical subject headings (MeSH) and text words for esophagogastric cancer and for each treatment option were used in the search as described previously [Citation10] (Supplementary Table 1). Two independent reviewers (EtV and JJvK) were involved in screening the titles, abstracts and full-texts. Disagreements were discussed with a third arbiter (HvL) until consensus was reached.
Study selection and quality assessment
We selected RCTs that included at least one treatment arm with first-line palliative chemotherapy containing currently used compounds for patients with locally irresectable or metastatic esophagogastric cancer: fluoropyrimidines (i.e., 5-fluorouracil [5-FU], capecitabine or S-1), platinum (i.e., cisplatin or oxaliplatin), anthracycline (i.e., epirubicin), taxanes (i.e., docetaxel or paclitaxel) and irinotecan. In addition, any data on toxicity in the RCTs was scored according to the Common Terminology Criteria for Adverse Events (CTCAE) and was reported using the maximum grade method (i.e., only the highest occurring grade of an adverse event is registered per patient) [Citation11].
Critical study appraisal was performed with the Cochrane Risk of bias tool (version 5.1.0) [Citation12] and was done by two authors independently (EtV and LN). Items were scored as low, high or unknown risk of bias.
Data extraction and statistical analysis
From each separate chemotherapy arm in RCTs, AE incidence counts and the total number of patients in the toxicity population were extracted from the toxicity tables or from the text in the study reports. We divided the AEs into mild (grade 1 or 2), severe (grade 3 or 4) and fatal (grade 5). As the majority of the RCTs solely reported the AEs that occurred in ≥5% of the patients, we extracted a pre-specified set of common chemotherapy AEs, in order to standardize the extraction of AEs for all chemotherapy regimens and to keep the TOXviews comparable as CTCAE is composed of numerous categories and not every adverse event is present in each regimen due the maximum grade method of reporting.
Statistical analyses were performed with the metafor package [Citation13] (version 2.0-0) in R (version 3.5.1) [Citation14]. From the extracted AE incidence counts and toxicity populations, pooled proportions and 95% confidence intervals (95%CI) were calculated with a single-arm proportional random-effects meta-analysis following a logit transformation. To graphically present the toxicity profiles of each chemotherapy regimen in a TOXview, the pooled proportions of AE-incidences were summarized in bar charts, along with the 95%CI. Tables containing the number of events, total number of participants, number of trials and pooled estimates were constructed and shown alongside the bar chart. In accordance with a network meta-analysis reported previously, TOXviews for the following regimens were constructed: fluoropyrimidine-alone, cisplatin-containing doublets (C-doublets), non-cisplatin fluoropyrimidine-containing doublets (F-doublets), anthracyclin-containing triplets (A-triplets) and taxane-containing triplets (T-triplets) [Citation10].
To validate the data presented in the TOXviews, we performed a comparative analysis of incidences of AEs between regimens based on our previous work [Citation10] using the Wald test as overall test and additional two-sided post-hoc tests, with Holms correction for multiple comparisons. All tests had a significance level of p = .05. To display the comparisons in a graphical presentation, we constructed a bidirectional chart of TOXviews.
Results
Description of the included studies
Until February 2017, a total of 5765 unique titles were retrieved through the database search, of which 191 RCTs remained after screening the titles and abstracts. After full-text assessment, 108 were excluded and 83 were found eligible. In addition, 9 studies retrieved from the conference search were found eligible (Supplementary Figure 1). A total of 92 studies containing 16,963 patients were included [Citation15–106]. No major differences in study design were observed (Supplementary Table 2). Forty-four (48%) studies were rated as having a low risk of bias (Supplementary Figure 2). Fourteen (15%) and 18 (20%) studies were rated as unclear risk of bias on only one item or two items, respectively. The other 16 studies (17%) were rated as unclear on three items or were reported as abstract only.
Presentation of TOXview of singlet, doublet, triplet cytotoxic regimens
It was possible to construct individual toxicity profiles for twenty-eight cytotoxic regimens and these were graphically represented in TOXviews (Supplementary material). As stated before, we constructed TOXviews for regimens including cytotoxic compounds that are used nowadays in the treating AEGC and which are potentially clinically relevant chemotherapy regimens according to previous work [Citation10]. Two types of TOXviews were presented, using the toxicity profile of the doublet oxaliplatin-capecitabine as an example: one that consists of a table and a bar chart with confidence intervals and one that is displayed as a bidirectional chart with confidence intervals (). TOXviews for chemotherapy regimens were divided into different groups: cytotoxic single agent regimes, cytotoxic doublet regimens, cytotoxic triplet regimens () which were used for further comparative analysis.
Figure 1. Example for TOXview. Toxicity profile of capecitabine (CAP)+oxaliplatin (Ox) (CAPOX) presented in two different ways. (A) Barchart and table CAPOX. (B) Bidirectional chart and table CAPOX. Grade 1–2 left directed, grade 3–4 right directed, a grade 5 adverse event (fatal) was represented as toxicity related death.
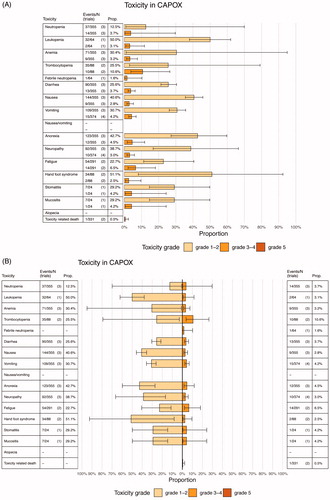
Figure 2. Overview of chemotherapy regimens used for TOXview. (A) Cytotoxic singlet regimens (B) Cytotoxic doublet regimens (C) Cytotoxic triplet regimens RCT: randomized controlled trials; 5-FU: 5-fluorouracil; CAP: capecitabine; C: cisplatin; D or DTX: docetaxel; I: irinotecan; PTX: paclitaxel; Ox: oxaliplatin; T: taxane; F: fluoropyrimidine; A: anthracycline; FAMTX: 5-FU + doxorubicin + methotrexate; FEMTX: 5-FU + epirubicin + methotrexate; ECX: epirubicin + cisplatin + capecitabine; ECF: epirubicin + cisplatin + 5-FU; FOXP: 5-FU + oxaliplatin + paclitaxel; FOXD: 5-FU + oxaliplatin + docetaxel; EOF: epirubicin + oxaliplatin + 5-FU; EOX: epirubicin + oxaliplatin + capecitabine.
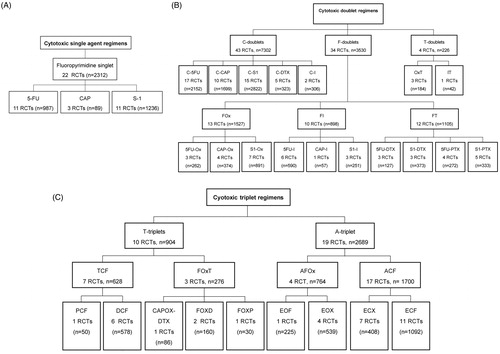
Comparative analysis of TOXviews
C-doublets were compared to F-doublets as shown in the bidirectional plots and table (; ). To show the differences among the F-doublet regimens, C-doublets were also compared to separate F-doublets (Supplementary Figure 3). Next, F-doublets were compared against T-triplets and A-triplets (; ). In general, F-doublets showed more grade 1–2 but less grade 3–4 differences in incidences of adverse events compared to C-doublets. Compared to F-doublets, A-triplets and T-triplets showed more grade 3–4 adverse events and toxicity-related-deaths. When A and T-triplets were compared, T-triplets showed more adverse events than A-triplets.
Figure 3. Bidirectional comparison plot for C-doublets versus F-doublets. C: Cisplatin. F: Fluoropyrimidine.
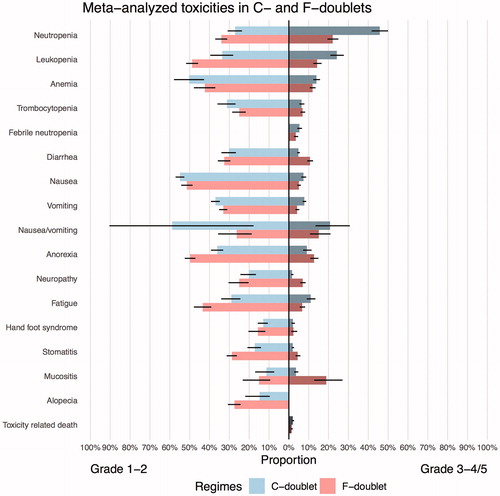
Figure 4. Bidirectional comparison plot for A-triplets versus T-triplets versus F-doublets. A: anthracycline; T: Taxane; F: Fluoropyrimidine.
Table 1. Statistically significant differences between incidences of chemotherapy regimens (p < .05) C-doublets versus F-doublets.
Table 2. Statistically significant differences between incidences of chemotherapy regimens (p < .05). F-doublets versus A-triplets versus T-triplets.
Heterogeneity
High heterogeneity numbers were detected for a majority of single-arm meta-analyses. In the individual TOXviews in which the portion of heterogeneity was statistically significant for any given AE according to the Q-test, the I2 of these individual TOXviews ranged from 47% to 99%. In the comparative analysis, the statistically significant heterogeneity in I2 ranged from 54% to 97%.
Discussion
This study introduces a new graphical method to present toxicity of systemic therapies by using single arm pooled proportions meta-analysis. With TOXview, we constructed a method where we can illustrate the effect of an intervention and its confidence interval. With single arm pooled proportions meta-analysis, we combined adverse events while taking into account each confidence interval of the adverse event in a specific study. We thereby could estimate the pooled proportion with its CI with a random effects model. Moreover, by adding information about the events, the total number of participants and the number of trials, it is possible to interpret the confidence interval of the pooled proportion.
Up to now, there was no uniformly utilized graphical presentation format of AE to use during a consultation. With the lack of an accurate toxicity overview, it can be difficult for physicians to provide realistic risks of adverse events while discussing systemic treatment options. A graphical presentation, such as TOXview, could be a convenient tool to improve accurate communication by physicians about toxicity and to improve understanding of patients about the expected toxicity of specific treatment regimens [Citation3,Citation4]. With TOXview, it is possible for both the physicians and patient to see how the toxicities are distributed per chemotherapy regimen. As the incidence and confidence intervals are given, physicians can make better estimations of the potential tolerability for an individual patient and can provide detailed information about the nature of adverse events that patients can expect with a certain regimen. We have used palliative first-line chemotherapy for AEGC as an example. According to our previous work [Citation10], C-doublets were less effective and had increased toxicity compared to F-doublets whereas A-triplets and taxane + cisplatin + fluoropyrimidine (TCF) were not more effective and had increased toxicity over F-doublets. Fluoropyrimidine + Oxaliplatin + Taxane (FOxT) was found more effective but had increased toxicity compared to F-doublets. Also from the analysis of the TOXviews, we can conclude that F-doublets are the preferable chemotherapy regimen based on its low incidence of high-grade toxicities. Compared to F-doublets, C-doublets, A-triplets and T-triplets had higher incidences of severe adverse events. This shows that the data used in the TOXviews collected from single arms in different RCTs is in concordance with the results of our previous work based on between-arm comparisons of toxicities [Citation10].
Thanarajasingnam et al. [Citation4] published a new graphical method as well, based on individual patient data and incorporated time in the toxicity presentation. We are aware that our presentation of toxicity does not provide any information to compare toxicity over time between regimens, even though this may be an important feature to share with patients when choosing a chemotherapy regimen. Unfortunately, in most clinical studies, the maximum-grade method is used to report data about toxicity and time to event is usually not incorporated [Citation11]. To make a better estimation of the incidences of toxicities, analysis of individual patient data would preferably incorporate time to event. This also opens the opportunity to study multiple events per patient over time.
TOXview also has some limitations. Firstly, high heterogeneity numbers were observed in the TOXviews, indicating substantial variation between studies. This was partly expected because data of single arms from RCTs were used. Moreover, different dosages of chemotherapy and the intensity of cycles could explain the heterogeneity numbers in our data. Due to high heterogeneity, all analyses were performed with a random-effects model. In addition, as described before by Sivendran et al. [Citation11], possible causes of the high heterogeneity could be unawareness of systematically reporting adverse events, inadequate compliance to protocols and selective reporting. It is difficult for patients to notice all aspects of their adverse event and report it back to their physicians as not every physician score the same for each adverse event [Citation107]. It has been shown that documentation of adverse events in daily out-patient clinical practice is difficult for several reasons, such as unclear adverse events scoring terms, few experiences of scoring toxicity and the difficulty of the small difference between toxicity related adverse events or disease-related events [Citation11,Citation107,Citation108]. Up to now, the most accurate documentation of toxicities is done in clinical trials where a standard protocol is followed to administrate adverse events systematically. This can also contribute to a different estimation of the number of adverse events. Besides the heterogeneity caused by possible factors described as before, TOXviews have the limitation that confidence intervals are dependent on the number of studies and patients. In , we chose to show capecitabine + oxaliplatin (CAPOX), because this regimen is frequently used in clinical practice. This TOXview contains a maximum of four studies, thereby contributing to a wide CI. Other frequently used regimens that were based on many more studies and therefore, show less wide confidence intervals.
Another limitation of this study is that most studies only reported AEs in the study report when these reached a threshold of e.g., 5% of the total group [Citation11]. This could imply that some adverse events are underreported in the TOXviews even though they were present. To counter this problem, we used a prespecified set of common AEs of chemotherapy; as such the most important AEs could be compared between the regimens. In addition, very few studies explicitly mention that the adverse events are possibly related to treatment, which could also result in underreported adverse events [Citation41,Citation64]. For future research, using individual patient data or creating an international adverse event report system e.g. PRO-CTCAE™ [Citation109] could create better estimation of the incidences in the TOXview and adverse events in general.
Conclusion
TOXview is the first attempt to present a pooled overview of systemic treatment toxicities from multiple studies. We advocate TOXview as a new tool in the clinic to promote shared decision making of physicians with their patients. Particularly regarding AEGC, for which no formally golden standard first-line palliative chemotherapy exists, TOXview could provide better guidance in communicating expected toxicities and may promote shared decision-making between patient and physician.
Supplemental Material
Download MS Word (7.3 MB)Disclosure statement
H.W.M.v.L. served as a consultant for Celgene, Lilly, and Nordic. H.W.M.v.L: received funding outside the submitted work from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips and Roche. M.G.H.v.O received funding outside the submitted work from Bayer, Lilly, Merck Serono and Roche; all other authors declare that they have no potential conflicts of interest.
References
- Pinto E, Cavallin F, Saadeh LM, et al. Potential curability and perception of received information in esophageal cancer patients. Support Care Cancer. 2018;26:1807–1814.
- Faller H, Koch U, Brähler E, et al. Satisfaction with information and unmet information needs in men and women with cancer. J Cancer Surviv. 2016;10:62–70.
- Mendoza T. Time for better presentation and analysis of adverse events. Lancet Oncol. 2016;17:553–554.
- Thanarajasingam G, Atherton PJ, Novotny PJ, et al. Longitudinal adverse event assessment in oncology clinical trials: the Toxicity over Time (ToxT) analysis of Alliance trials NCCTG N9741 and 979254. Lancet Oncol. 2016;17:663–670.
- Yeh S-T. Clinical adverse events data analysis and visualization. The Pharmaceutical industry SAS users group conference proceedings; Denver, CO: GlaxoSmithKline, 2007. PO10.
- ter Veer E, Ngai LL, van Valkenhoef G, et al. Capecitabine, 5-fluorouracil and S-1 based regimens for previously untreated advanced oesophagogastric cancer: a network meta-analysis. Sci Rep. 2017;7:3–10.
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67:7–30.
- Wagner A, Syn N, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Data Syst Rev. 2017;8:CD004064. DOI: 10.1002/14651858.CD004064.pub4
- Al-Batran SE, Van Cutsem E, Oh SC, et al. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol. 2016;27:673–679.
- Veer ET, Mohammad NH, Van Valkenhoef G, et al. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: a network meta-analysis. J Natl Cancer Inst. 2016;108:1–13.
- Sivendran S, Latif A, McBride RB, et al. Adverse event reporting in cancer clinical trial publications. JCO. 2014;32:83–89.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Cochrane Collab. 2011. Available from: http://handbook.cochrane.org
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
- R Core Team. R: a language and environment for statistical computing. R Found Stat Comput. 2018. Vienna, Austria. Available online at https://www.R-project.org/.
- Lu Y, Liu Z, Zhang J. S-1 plus oxaliplatin vs. S-1 as first-line treatment in patients with previously untreated advanced gastric cancer: a randomized phase II study. J Chemother. 2014;26:159–164.
- Yun J, Lee J, Park SH, et al. A randomised phase II study of combination chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or cisplatin and capecitabine (CX) in advanced gastric cancer. Eur J Cancer. 2010;46:885–891.
- Van Cutsem E, Boni C, Tabernero J, et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann Oncol. 2015;26:149–156.
- Thuss-Patience PC, Kretzschmar A, Repp M, et al. Docetaxel and continuous-infusion fluorouracil versus epirubicin, cisplatin, and fluorouracil for advanced gastric adenocarcinoma: a randomized phase II study. JCO. 2005;23:494–501.
- Tesselaar ME, Luelmo S, Polee M, et al. Randomized, phase II study comparing cisplatin and high-dose 5-fluorouracil/leucovorin with paclitaxel and high-dose 5-fluorouracil/leucovorin in patients with advanced gastric cancer and adenocarcinomas of the gastroesophageal junction. JCO. 2008;26:4567.
- Tebbutt NC, Norman A, Cunningham D, et al. A muticentre, randomised phase III trial comparing protracted venous infusion (PVI) 5-fluorouracil (5-FU) with PVI 5-FU plus mitomycin C in patients with inoperable oesophago-gastric cancer. Ann Oncol. 2002;13:1568–1575.
- Sym SJ, Hong J, Park J, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol. 2013;71:481–488.
- Sugimoto N, Fujitani K, Imamura H, et al. Randomized phase II trial of S-1 plus irinotecan versus S-1 plus paclitaxel as first-line treatment for advanced gastric cancer (OGSG0402). Anticancer Res. 2014;34:851–857.
- Shirao K, Boku N, Yamada Y, et al. Randomized phase iii study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (jcog0106). Jpn J Clin Oncol. 2013;43:972–980.
- Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. 2015;18:168–176.
- Shah MA, Janjigian YY, Stoller R, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US gastric cancer consortium. JCO. 2015;33:3874–3879.
- Sawaki a, Yamaguchi K, Nabeya Y, et al. 5-FU/l-LV (RPMI) versus S-1 as first-line therapy in patients with advanced gastric cancer: a randomized phase III non-inferiority trial. (ISO-5FU10 Study Group trial). Eur J Cancer Suppl. 2009;7:364.
- Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol. 2016;27:2196–2203.
- Ryu M-H, Park YI, Chung I-J, et al. Phase III trial of s-1 plus oxaliplatin (SOX) vs s-1 plus cisplatin (SP) combination chemotherapy for first-line treatment of advanced gastric cancer (AGC): SOPP study. JCO. 2016;34:4015.
- Ryu MH, Baba E, Lee KH, et al. Comparison of two different S-1 plus cisplatin dosing schedules as first-line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol. 2015;26:2097–2101.
- Roy A, Cunningham D, Hawkins R, et al. Docetaxel combined with irinotecan or 5-fluorouracil in patients with advanced oesophago-gastric cancer: a randomised phase II study. Br J Cancer. 2012;107:435–441.
- Roth AD, Fazio N, Stupp R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss group for clinical cancer research. JCO. 2007;25:3217–3223.
- Roth A, Kolaric K, Zupanc D, et al. High doses of 5-fluorouracil and epirubicin with or without cisplatin in advanced gastric cancer: a randomized study. Tumori. 1999;85:234–238.
- Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. JCO. 2002;20:1996–2004.
- Ridwelski K, Fahlke J, Kettner E, et al. Docetaxel-cisplatin (DC) versus 5-fluorouracil-leucovorin-cisplatin (FLC) as first-line treatment for locally advanced or metastatic gastric cancer: preliminary results of a phase III study. JCO. 2008;26:4512.
- Richards D, Kocs DM, Spira AI, et al. Results of docetaxel plus oxaliplatin (DOCOX) ± cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: Results of a randomised Phase 2 study. Eur J Cancer. 2013;49:2823–2831.
- Rao S, Starling N, Cunningham D, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol. 2010;21:2213–2219.
- Pozzo C, Barone C, Szanto J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: Results of a randomized phase II study. Ann Oncol. 2004;15:1773–1781.
- Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148.
- Park SH, Lee WK, Chung M, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs. 2006;17:225–229.
- Ocvirk J, Reberšek M, Skof E, et al. Randomized prospective phase II study to compare the combination chemotherapy regimen epirubicin, cisplatin, and 5-fluorouracil with epirubicin, cisplatin, and capecitabine in patients with advanced or metastatic gastric cancer. Am J Clin Oncol. 2012;35:237–241.
- Ochenduszko S, Puskulluoglu M, Konopka K, et al. Comparison of efficacy and safety of first-line palliative chemotherapy with EOX and mDCF regimens in patients with locally advanced inoperable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma: a randomized phase 3 trial. Med Oncol. 2015;32:1–8.
- Nishikawa K, Morita S, Matsui T, et al. A randomized phase-II trial comparing sequential and concurrent paclitaxel with oral or parenteral fluorinated pyrimidines for advanced or metastatic gastric cancer. Gastric Cancer. 2012;15:363–369.
- Nio Y. A randomized, comparative study of combination chemotherapies in advanced gastric cancer: 5-Fluorouracil and cisplatin (FP) versus 5-Fluorouracil, cisplatin, and 4’-Epirubicin (FPEPIR). Anticancer Res. 1992;12:1983–1988.
- Narahara H, Iishi H, Imamura H, et al. Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer. 2011;14:72–80.
- Moehler M, Kanzler S, Geissler M, et al. A randomized multicenter phase II study comparing capecitabine with irinotecan or cisplatin in metastatic adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2010;21:71–77.
- Moehler M, Eimermacher A, Siebler J, et al. Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer. 2005;92:2122–2128.
- Mochiki E, Ogata K, Ohno T, et al. Phase II multi-institutional prospective randomised trial comparing S-1paclitaxel with S-1cisplatin in patients with unresectable and/or recurrent advanced gastric cancer. Br J Cancer. 2012;107:31–36.
- Lutz MP, Wilke H, Wagener DJT, et al. Weekly infusional high-dose fluorouracil (HD-FU), HD-FU plus folinic acid (HD-FU/FA), or HD-FU/FA plus biweekly cisplatin in advanced gastric cancer: Randomized phase II trial 40953 of the European organisation for research and treatment of cancer gastroi. JCO. 2007;25:2580–2585.
- Wu D, Li X, Tong J, et al. S-1 combined with cisplatin versus cisplatin alone for the treatment of advanced gastric cancer: a pilot randomized-controlled trial. Anticancer Drugs. 2015;26:774–778.
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499.
- Loehrer PJ, Harry D, Chlebowski RT. 5-Fluorouracil vs. epirubicin vs. 5-fluorouracil plus epirubicin in advanced gastric carcinoma. Invest New Drugs. 1994;12:57–63.
- Li Y, Qiu M, Xu J, et al. S-1 plus Cisplatin versus fluorouracil plus cisplatin in advanced gastric or gastro-esophageal junction adenocarcinoma patients: a pilot study. Oncotarget. 2015;6; 35107–35115.
- Li X-D, Shen H, Jiang J-T, et al. Paclitaxel based vs oxaliplatin based regimens for advanced gastric cancer. World J Gastroenterol. 2011;17:1082–1087.
- Lee KH, Hyun MS, Kim H-K, et al. Randomized, multicenter, phase III trial of heptaplatin 1-hour infusion and 5-fluorouracil combination chemotherapy comparing with cisplatin and 5-fluorouracil combination chemotherapy in patients with advanced gastric cancer. Cancer Res Treat. 2009;41:12.
- Konings I, Van Der Gaast A, Van Der Wijk LJ, et al. The addition of pravastatin to chemotherapy in advanced gastric carcinoma: a randomised phase II trial. Eur J Cancer. 2010;46:3200–3204.
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221.
- Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;99:584–590.
- Kobayashi M, Tsuburaya A, Nishikawa K, et al. A randomized phase II trial of capecitabine plus cisplatin (XP) versus S-1 plus cisplatin (SP) as a first-line treatment for advanced gastric cancer: XP ascertainment versus SP randomized PII trial (XParTS II). JCO. 2015;33:105.
- Kim ST, Kang JH, Lee J, et al. Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: a double-blind randomised phase 3 study. Eur J Cancer. 2014;50:2822–2830.
- Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. JCO. 1997;15:261–267.
- Kim GM, Jeung H-C, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer. 2012;48:518–526.
- Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673.
- Jeung H-C, Rha SY, Im CK, et al. A randomized phase 2 study of docetaxel and S-1 versus docetaxel and cisplatin in advanced gastric cancer with an evaluation of SPARC expression for personalized therapy. Cancer. 2011;117:2050–2057.
- Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–1018.
- Ikeda R, Yoshida K, Satou Y, et al. Randomized phase II/III study of docetaxel/S-1 (DS-1) versus CDDP/5FU (FUP) in advanced or recurrent gastric cancer: updated phase II results. J Clin Oncol. 2009;27:4595.
- Icli F, Celik I, Aykan F, et al. A randomized Phase III trial of etoposide, epirubicin, and cisplatin versus 5-fluorouracil, epirubicin, and cisplatin in the treatment of patients with advanced gastric carcinoma. Turkish Oncology Group. Cancer. 1998;83:2475–2480.
- Hwang IG, Ji JH, Kang JH, et al. A multi-center, open-label, randomized phase III trial of first-line chemotherapy with capecitabine monotherapy versus capecitabine plus oxaliplatin in elderly patients with advanced gastric cancer. J Geriatr Oncol. 2017;8:170–175.
- Huang D, Ba Y, Xiong J, et al. A multicentre randomised trial comparing weekly paclitaxel + S-1 with weekly paclitaxel + 5-fluorouracil for patients with advanced gastric cancer. Eur J Cancer. 2013;49:2995–3002.
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC - A randomized phase III trial. JCO. 2016;34:443–451.
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. JCO. 2011;29:3968–3976.
- Wang J, Xu R, Li J, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19:234–244.
- Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive. JCO. 2014;32:3520–3526.
- Fujitani K, Yang H-K, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–318.
- Eatock MM, Tebbutt NC, Bampton CL, et al. Phase II randomized, double-blind, placebo-controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann Oncol. 2013;24:710–718.
- Du F, Zheng Z, Shi S, et al. S-1 and cisplatin with or without nimotuzumab for patients with untreated unresectable or metastatic gastric cancer. Medicine. 2015;94:e958.
- Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457.
- Dai X, Zhang X, Wang C, et al. Paclitaxel/oxaliplatin/fluorouracil (TOF) regimen versus S-1/oxaliplatin (SOX) regimen for metastatic gastric cancer patients. Oncotarget. 2017;8:30495–30501.
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
- Coombes RC, Chilvers CE, Amadori D, et al. Randomised trial of epirubicin versus fluorouracil in advanced gastric cancer. An International Collaborative Cancer Group (ICCG) study. Ann Oncol Off J Eur Soc Med Oncol. 1994;5:33–36.
- Colucci G, Giotta F, Maiello E, et al. Efficacy of the association of folinic acid and 5-fluorouracil alone versus folinic acid and 5-fluorouracil plus 4-epidoxorubicin in the treatment of advanced gastric carcinoma. Am J Clin Oncol Cancer Clin Trials. 1995;18:519–524.
- Cocconi G, Carlini P, Gamboni A, et al. Cisplatin, epirubicin, leucovorin and 5-fluorouracil (PELF) is more active than 5-fluorouracil* Doxorubicin and methotrexate (FAMTX) in advanced gastric carcinoma. Ann Oncol. 2003;14:1258–1263.
- Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489.
- Cocconi G, Bella M, Zironi S, et al. Fluorouracil, doxorubicin, and mitomycin combination versus PELF chemotherapy in advanced gastric cancer: a prospective randomized trial of the Italian Oncology Group for Clinical Research. JCO. 1994;12:2687–2693.
- Bouché O, Raoul JL, Bonnetain F, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Fédération Francophone de Cancérologie. JCO. 2004;22:4319–4328.
- Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697.
- Bando H, Yamada Y, Tanabe S, et al. Efficacy and safety of S-1 and oxaliplatin combination therapy in elderly patients with advanced gastric cancer. Gastric Cancer. 2016;19:919–926.
- Al-Batran S-E, Pauligk C, Homann N, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer. 2013;49:835–842.
- Al-Batran S-E, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442.
- Komatsu Y, Takahashi Y, Kimura Y, et al. Randomized phase II trial of first-line treatment with tailored irinotecan and S-1 therapy versus S-1 monotherapy for advanced or recurrent gastric carcinoma (JFMC31-0301). Anticancer Drugs. 2011;22:576–583.
- Wang X, Wang ML, Zhou LY, et al. Randomized phase II study comparing paclitaxel with S-1 vs. S-1 as first-line treatment in patients with advanced gastric cancer. Clin Transl Oncol. 2013;15:836–842.
- Yoshino S, Nishikawa K, Morita S, et al. Randomised phase III study of S-1 alone versus S-1 plus lentinan for unresectable or recurrent gastric cancer (JFMC36-0701). Eur J Cancer. 2016;65:164–171.
- Vanhoefer U, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: atrial of the Europe. JCO. 2000;18:2648–2657.
- Hironaka S, Sugimoto N, Yamaguchi K, et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:99–108.
- Hall PS, Lord SR, Collinson M, et al. A randomised phase II trial and feasibility study of palliative chemotherapy in frail or elderly patients with advanced gastroesophageal cancer (321GO). Br J Cancer. 2017;116:472–478.
- Kondo K, Sakamoto J, Nakazato H, et al. A phase III randomized study comparing doxifluridine and 5-fluorouracil as supportive chemotherapy in advanced and recurrent gastric cancer. Oncol Rep. 2000;7:485–490.
- Ajani JA, Abramov M, Bondarenko I, et al. A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann Oncol. 2017;28:2142–2148.
- Al-Batran S-E, Schuler MH, Zvirbule Z, et al. FAST: an international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gast. JCO. 2016;34:LBA4001–LBA4001.
- Ohtsu A, Shimada Y, Shirao K, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: the Japan clinical oncology group study (JCOG9205). JCO. 2003;21:54–59.
- Jin M, Lu H, Li J, et al. Ramdomized 3-armed phase III study of S-1 monotherapy versus S-1/CDDP (SP) versus 5-FU/CDDP (FP) in patients (pts) with advanced gastric cancer (AGC): SC-101 study. JCO. 2008;26:4533.
- Koizumi W, Yamaguchi K, Hosaka H, et al. Erratum: randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer (British Journal of Cancer (2013) 109 (2079-2086). Br J Cancer. 2014;111:2382.
- Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. JCO. 2010;28:1547–1553.
- Ajani J. A, Fodor MB, Tjulandin S. a, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. JCO. 2005;23:5660–5667.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. JCO. 2006;24:4991–4997.
- Park SH, Nam E, Park J, et al. Randomized phase II study of irinotecan, leucovorin and 5-fluorouracil (ILF) versus cisplatin plus ILF (PILF) combination chemotherapy for advanced gastric cancer. Ann Oncol. 2007;19:729–733.
- Zhang ZD, Kong Y, Yang W, et al. Clinical evaluation of cetuximab combined with an S-1 and oxaliplatin regimen for Chinese patients with advanced gastric cancer. World J Surg Oncol. 2014;12:1–6.
- Sivendran S, Galsky MD. Adverse event reporting in oncology clinical trials - lost in translation? Expert Opin Drug Saf. 2016;15:893–896.
- Zhang S, Chen Q, Wang Q. The use of and adherence to CTCAE v3.0 in cancer clinical trial publications. Oncotarget. 2016;7:65577–65588.
- Schoen MW, Basch E, Hudson LL, et al. Software for administering the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events: usability study. JMIR Hum Factors. 2018;5:e10070.
