Abstract
Background: Phase II trials are designed to assess the efficacy/toxicity ratio of experimental treatments and select those worth being tested in phase III trials. Although crucial limitations were identified when concurrent chemoradiation (cCRT) phase III trials characteristics were assessed, features of cCRT phase II trials have never been reported. The objective was to describe features of all cCRT phase II trials.
Methods and material: Requests were performed in the Medline database (via PubMed). The latest update was performed in April 2016, using the following MESH terms: ‘clinical trials: phase II as topic’, ‘chemoradiotherapy’.
Results: Four hundred and fifty-eight cCRT phase II trials were identified. They were mainly multicenter (51.5%), single arm studies (77.7%) published after 2011 (55.0%). The median number of included patients was 52. Primary endpoints were mainly response rate (20.5%), pathological complete response (14.4%) and overall survival (12.6%). The primary endpoint was not defined in 22% of studies. Tumors were mostly lung (23.1%), head and neck (20.3%), colorectal (16.6%) and esophagogastric cancer (14.6%) treated at a locally advanced setting (81.7%). 55.2% of trials used 3D-conformal radiotherapy and 9.1% intensity-modulated radiotherapy, mainly with normo-fractionation (82.0% of the 573 arms with radiotherapy). Radiation technique was not reported in 19.9% of studies. Associated anticancer drugs (563 arms) were mainly conventional chemotherapies (559 arms): cisplatin (46.2%) and 5-fluorouracil (28.3%). Non cytotoxic agents (targeted therapies, immunotherapies) were tested in 97 arms (17%). With a median follow-up of 31 months, acute grades 3–5 were reported in 98.5% of studies and late toxicities in 44.5%. Follow-up was not reported in 17% of studies.
Conclusions: cCRT phase II trials featured severe limitations, with outdated radiation techniques, insufficient reporting of crucial data and a small number of included patients. This certainly limited the impact of conclusions and hindered the development of successful phase III trials.
Introduction
In the past decade, the failure rate of phase III radiotherapy trials has been disappointingly high [Citation1]. Out of hundreds of studies testing anticancer drugs combined with radiotherapy, only three finally obtained a marketing authorization: temozolomide for glioblastoma, cetuximab for head and neck squamous cell carcinoma, and durvalumab (after chemoradiotherapy) for stage III non-small cell lung carcinoma. In a comprehensive review of randomized phase III clinical trials including at least radiation, the present team highlighted crucial limitations regarding the methodology and the characteristics of such studies [Citation2]. Radiation techniques were outdated in the vast majority of trials and the quality of reporting was questionable. Radiotherapy technique, follow-up and late toxicities were not reported in 30–40% of publications. Consequently, the quality of information collected in earlier phase radiotherapy studies was questioned too. The fate of pharmaceutical agents tested with radiation (such as gemcitabine, bevacizumab or tirapazamine) could have arguably been different had modern radiation techniques been performed as part of a step-by-step clinical development relying on quality assurance as well as a proper assessment of toxicities [Citation3]. However, to our best knowledge, an exhaustive literature review describing the major characteristics of cCRT phase II trials has never been carried out. Yet, phase II trials should play a pivotal role as theoretically they limit access to long, complex and expensive phase III trials only to the most promising experimental treatments.
The aim of the present study was therefore to describe and analyze all cCRT phase II clinical trials.
Methods
Requests were performed in the Medline database (via PubMed) to identify all publications about phase II trials analyzing concomitant chemoradiotherapy between 1993 (first cCRT phase II) and 2016. The latest update was performed in April 2016, using the following MESH terms: ‘clinical trials: phase II as topic’, ‘chemoradiotherapy’, as keywords and ‘English’ as limit.
Study selection
Phase II studies were eligible for inclusion if cancer patients (including malignant blood diseases) were prospectively recruited and if at least, one of the assigned treatments included a chemotherapy concurrently performed with radiotherapy. Exclusion criteria were: phase I trials, phase III trials, phase II trials testing sequential chemoradiotherapy, absence of available full text and sole abstracts. In case of several publications for the same trial, only the most recent data were considered. A first selection was based on title and abstract. Then, eligible articles were selected on full text and reviewed. Selections were carried out independently by two reviewers. Concordant articles were included in analysis by the first reviewer and disagreements between the two selections were resolved by a third reviewer.
Data collection
For each selected trial, two different reviewers collected the following data: the name of the journal, year of publication, number of participating centers (mono/multicenter study), design of the study (randomized vs. non-randomized), number of treatment arms, median follow up, primary endpoint (response rate, pathological complete response, overall survival, progression-free survival, disease-free survival, toxicity, etc.), number of included patients, cancer type, stage (early stage, locally advanced, metastatic setting), radiotherapy fractionation (standard fractionation: 1.8–2.2 Gy), radiotherapy technique, radiation dose escalation (yes/no), the name of the concurrent chemotherapy, concurrent non-cytotoxic agent (NCA: including targeted therapies, immunotherapies, hormonotherapies and other agents such as radiosensitizing or radioprotective agents, etc.) administration (yes/no), NCA name, acute all grades and acute grades 3–5 toxicities (yes/no, evaluated by the National Cancer Institute Common Toxicity Criteria (CTCAE v3.0 )), late (>3 months) toxicities (yes/no), quality of life (yes/no) and industrial sponsorship.
Results
Literature selection
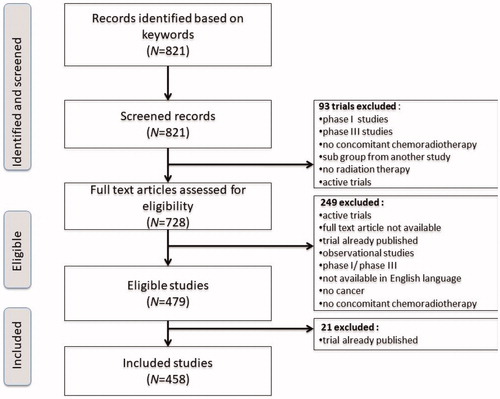
Initial research in Medline database resulted in 821 hits. After a first selection, based on title and abstract, 728 full-text reports were assessed for eligibility. After removing duplicates, 458 studies met inclusion and exclusion criteria and were analyzed ().
Studies characteristics
Data about 458 cCRT phase II trials were collected. Fifty-five percent were from recent publications (2011–2016). Most studies were published in four journals: International Journal of Radiation Oncology-Biology-Physics (17.2%), Journal of Clinical Oncology (11.8%), Radiotherapy and Oncology (6.1%) and Annals of Oncology (4.4%). Most phase II trials were multicenter (51.5%) single arm studies (77.7%). Out of the 102 studies with more than one arm, 71 were randomized (15.5% of all studies). The median number of patients included per study was 52 (IQR: 35–74). The main primary endpoints were the response rate (20.5%), the pathological complete response (14.4%) and the overall survival (12.6%). The primary endpoint was not defined in 22% of studies. The median follow-up was 31 months (21.6–44). It was not reported in 22% of studies. Funding sources were not available in 35.1% of publications. Studies characteristics are reported in .
Table 1. Characteristics of included studies (n = 458 studies).
Patients’ characteristics
The most studied primary locations were lung (23.1%), head and neck (20.3%), colorectal (16.6%) and esophagogastric cancer (14.6%). Patients mainly had locally advanced tumors (81.7%). Patients’ characteristics are reported location by location in .
Data about treatment
The 458 studies included 585 treatment arms among which 573 arms using radiotherapy. Radiation technique was 3D-conformal radiotherapy in 253 studies (55.2%), intensity-modulated radiotherapy (IMRT) in 42 studies (9.1%), 2D radiotherapy in 17 trials (3.7%) and featured brachytherapy in three studies (0.6%). Innovative radiotherapy was used in four studies (proton 0.4% and stereotactic body radiotherapy (SBRT): 0.4%). Different radiation techniques were accepted in 48 studies (10.4%). Radiotherapy technique was not described in 91 trials (19.9%).
Normo-fractionation (1.8–2.2 Gy/fraction, one fraction a day, five days a week) was performed in 474 arms (82.0%), hypofractionation (>2.2 Gy/fraction, one fraction every 24–48 h, 3–5 fractions a week) in 33 arms (5.8%), bifractionation (two fractions a day, five days a week) in 23 arms (4.0%) and hyperfractionation (>2 fractions a day, five days a week) in 20 arms (3.4%). A radiation dose-escalation was performed in 15 studies (3.2%). Radiotherapy characteristics are reported in .
Table 2. Radiotherapy characteristics (n = 585 arms).
Out of the 458 studies, an anticancer drug (chemotherapy or non-cytotoxic drug) was associated with radiation in 563 arms. Chemotherapy was concurrently associated with radiotherapy in 559 arms. Cisplatin (258 arms, 46.2%), 5-fluorouracil (158 arms, 28.3%), paclitaxel (75 arms, 13.4%), capecitabine (64 arms, 11.4%) and carboplatin (57 arms, 10.2%) were mainly prescribed. Chemotherapy characteristics are summarized in . A NCA was prescribed in 97 treatment arms. Main NCAs were targeted therapies (70 arms, 72.2%). NCA characteristics are detailed in .
Table 3. Cytotoxic and non-cytotoxic agents characteristics.
Follow-up, toxicities and quality of life
Median time to follow-up was 31 months (IQR: 21.6–44) but the information was not given for 78 studies (17%). The quality of life was evaluated in 45 studies (9.8%). Acute all-grade toxicities were reported in 445 studies (97.2%) and acute grades 3–5 toxicities in 451 studies (98.5%). Data about late toxicities were reported in 204 studies (44.5%). Data about the reporting of adverse events are in .
Table 4. Data about adverse event reporting (N = 458 studies).
Discussion
The present study is the first comprehensive analysis of concurrent chemoradiation (cCRT) phase II studies. With the growing number of developing anticancer drugs (chemotherapies, targeted therapies, immunotherapies, etc.), phase II trials should play a pivotal role in screening out toxic or inefficient experimental treatments. In a near future, they should also be cornerstones to validate biomarkers and achieve successful development of immune-modulatory radiotherapy trials that can be ruined by an inappropriate patients’ selection [Citation4]. Yet, the present study identified several major improvable points regarding the methodology and the quality of reporting in publications. The small number of included patients (median: 52 patients per study), the absence of a defined primary endpoint (22%), follow-up (22%), funding sources (35.1%), late toxicities (63.5%) or even radiation techniques (19.9%) in a significant proportion of studies probably restricted the possibilities to learn from these trials [Citation5,Citation6]. Furthermore, modern radiotherapy techniques (intensity modulated radiotherapy: 9.1%, stereotactic-body radiotherapy: 0.4%) were rarely performed. Such a discrepancy between daily-routine practice and literature certainly limited the impact of conclusions as well as the development of successful phase III trials. The last decade of radiotherapy research is considered as ‘10 years of comparative failure’ [Citation3]. It was probably partly due to an inadequate quality assurance, outdated radiotherapy techniques and poor toxicity assessments in radiotherapy trials [Citation2]. The present study confirms that phase II chemoradiation trials featured severe limitations.
A previous review of all phase I chemoradiation trials was performed based on similar inclusion criteria and included 228 trials [Citation7]. The present analysis included 458 trials. This suggests that the ‘phase I’ step was often skipped although it is necessary to move from laboratory to clinical research. Regarding phase 3 radiotherapy trials published at the same period as the present study, a chemotherapy was associated in 239 studies (55.6%), resulting in 424 arms. Cisplatin (214 arms, 50.5%), 5-fluorouracil (116 arms, 27.4%), carboplatin (48 arms, 11.3%) and etoposide (47 arms, 11.1%) were mainly prescribed [Citation2]. Modern radiotherapy techniques were rarely used (IMRT: 1.9%). The radiation technique was not reported in 39% of arms. Late toxicities were not reported in 70% of studies. These figures were not very different from those reported in the present review of phase II studies. This should question the methodological aspects of research in radiation oncology. It is as if phase II studies were continued in phase III randomized controlled trials using the same radiotherapy techniques, the same pharmacological agents, and featuring the same issues of quality assurance. This questionable clinical strategy in phases I and II is certainly partly responsible for the high failure rate reported in phase III trials for the last 10 years [Citation1]. However, stating that many opportunities have been lost and few phase III trials have been positive only as a result of underlying fragile phase II studies being forwarded as phase III studies is probably excessive. It could actually also reflect that there have been too few good alternatives to the studies done, such as other chemoradiation combinations. The choice of the primary endpoint could have been another key element to explain the number of missed opportunities: unlike phase III trials, overall survival was rarely selected as primary endpoint in phase II cCRT trials [Citation2]. Response and pathological complete response rates were mainly chosen as surrogate markers. The statistical significance can be reached earlier with response rate than with overall survival, which explained why median follow-up was shorter in this phase II review (median 31 months) than in phase III (median 50 months). The assessment of overall survival would have required a larger number of patients, a longer follow-up, and finally increased immediate costs. However, the results of the past 10 years in radiation phase III trials demonstrated that the correlation between the overall survival and its supposed surrogate markers does not seem so clear [Citation8,Citation9].
As to toxicity, phase II cCRT trials featured a high-quality reporting about acute adverse events with 97.2% reporting all-grade adverse events and 98.5% reporting high-grade adverse events. This was expected since the identification of the toxicity profile of a chemoradiation is crucial to select the patients fit enough to tolerate cCRT [Citation10]. Yet, late toxicity remains insufficiently explored as only less than half of studies reported it. In this era of personalized medicine and supportive care, the quality of life was often neglected since it was assessed only in 9.8%. Regarding radiation therapy characteristics, the present review reports the predominant use of 3D conformational radiotherapy in phase II cCRT trials (55.2%) whereas innovative techniques (IMRT, SBRT) are now commonly used in routine practice. Actually, very few trials were based on outdated concepts. In many cases, the radiotherapy details were representative of clinical practice at the time of study protocol development, even if they can be considered outdated from a later perspective. It was also the case in phase III radiotherapy trials [Citation2]. This situation highlights the fact that technological progress remains one step ahead of evidence-based medicine. This situation is a major problem in good prognoses (breast, prostate, etc.) or rare (cervix, sarcomas, etc.) cancers, since the radiation technique is often considered outdated when definitive results are published. Therefore, outcomes obtained with 3D conformational radiotherapy can hardly be extrapolated to intensity-modulated techniques, since major differences were observed, especially as far as toxicities were concerned [Citation11,Citation12].
Finally, the present comprehensive review of literature about cCRT phase II trials highlighted severe limitations. A significant proportion of publications did not report on late toxicities, radiation technique, follow-up and did not define the primary endpoint. The fast-paced evolution of radiation techniques created a discrepancy between literature and daily-routine practice. At the time of evidence-based medicine, too few chemoradiation phase III trials changed practice, probably partly due to deficient earlier phase trials. This suggests that the methodology of clinical research needs to be invented again. Phase II studies often have relatively short observation time, as they are aimed at tolerability and early evidence of effect on the tumor. It is legitimate to publish relatively data, but the trial study group should be obliged to do follow-up at a later stage, so that survival and long-term side effects can be addressed.
Disclosure statement
The authors report no conflict of interest.
References
- Liu X, Zhang Y, Tang L-L, et al. Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years. JAMA Oncol. 2018;4:1073–1079.
- Trone JC, Espenel S, Rehailia-Blanchard A, et al. Navigating the highlights of phase III trials: a watchful eye on evidence-based radiotherapy. Ann Oncol. 2017;28:2691–2697.
- Chargari C, Massard C, Deutsch E. Focus on the number of radiation oncology trials or on clinical relevance? JAMA Oncol. 2018;4:1791.
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712.
- Soares HP, Daniels S, Kumar A, et al. Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ. 2004;328:22–24.
- Gilbert A, Ziegler L, Martland M, et al. Systematic review of radiation therapy toxicity reporting in randomized controlled trials of rectal cancer: a comparison of patient-reported outcomes and clinician toxicity reporting. Int J Radiat Oncol Biol Phys. 2015;92:555–567.
- Rivoirard R, Vallard A, Langrand-Escure J, et al. Thirty years of phase I radiochemotherapy trials: latest development. Eur J Cancer. 2016;58:1–7.
- Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions and are they currently overused? BMC Med. 2017;15:134.
- Buyse M, Burzykowski T, Saad ED. The search for surrogate endpoints for immunotherapy trials. Ann Transl Med. 2018;6:231.
- Verma V, Simone CB, Werner-Wasik M. Acute and late toxicities of concurrent chemoradiotherapy for locally-advanced non-small cell lung cancer. Cancers. 2017;9:120.
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136.
- Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692.