In our hospital center, in primary oral chemotherapy prescriptions for high-risk patients, clinical pharmacists participate in the introduction of a treatment by analyzing the risk of interaction between the treatment and the patient’s entire drug list. Here, we present an adult, elderly, male patient with metastatic castration-resistant prostate cancer who received enzalutamide with significant comorbidities. Enzalutamide is a second-generation antiandrogen for treatment of advanced prostate cancer, and it has been specifically designed to bind and inhibit androgen receptors (AR). Prostate cancer cells remain dependent on AR signaling even in an androgen-deprived environment [Citation1]. Furthermore, enzalutamide may lead to significant clinical interactions owing to its enzyme-inducing effect. In this particular case, digoxin and fluindione were involved. Fluindione is a vitamin K antagonist (VKA) used to prevent thromboembolic complications related to certain atrial rhythm disorders in emboligenic heart disease. Digoxin is a cardiac glycoside, which increases myocardium contractility by direct activity. It may be used for certain supraventricular dysrhythmias, particularly atrial fibrillation.
Case report
A 72-year-old man was convened in late March 2018 for first-time prescription of enzalutamide at a dose of 160 mg as his prostate cancer treatment. Following medical consultation, as part of the pharmaceutical consultation that follows, many comorbidities were identified, including obesity, atrial fibrillation, hypertension, hiatal hernia, kidney tumor in remission, and prostate adenocarcinoma. These comorbidities were associated with polypharmacy, which included digoxin, fluindione, nebivolol, furosemide, potassium chloride, alprazolam, pantoprazole, and medical castration with leuprorelin.
Two alerts were transmitted to the oncologist concerning the risk of decreasing efficiency in presence of enzalutamide, with fluindione and digoxin, which have a narrow safety window. Moreover, digoxin is a substrate of the transport protein P-gp, which is also induced by enzalutamide; thus, enzalutamide could result in a larger decrease in digoxin plasma concentrations. The physician therefore accepted the pharmacist's proposal to replace VKA with low-molecular weight heparin (LMWH) administered once daily at a standard curative dose to avoid drug interaction and to guarantee thromboprophylaxis. However, because the patient refused LMWH injection, the oncologist was forced to continue fluindione treatment at a dose of 20 mg per day, with weekly monitoring of the international normalized ratio (INR). Regarding digoxin, a follow-up measurement of digoxinemia was recommended.
A cardiological consultation was then scheduled at approximately 1 week later to allow treatment adaptation. The cardiologist confirmed the continuation of fluindione, with close biological monitoring to maintain a therapeutic INR between 2 and 3. Continuation of nebivolol and termination of digoxin were also decided.
Several months later, to evaluate the effect of drug interaction with enzalutamide, a request was made with the patient's agreement to recover all the INR recorded in the city lab (). It appears that because of the introduction of enzalutamide, the patient’s INR had never been stabilized, and that the dosages of fluindione had been constantly modified.
Figure 1. Evolution of the patient’s INR and fluindione dosage. (A) Increased INR monitoring frequency. (B) Beginning of enzyme induction.
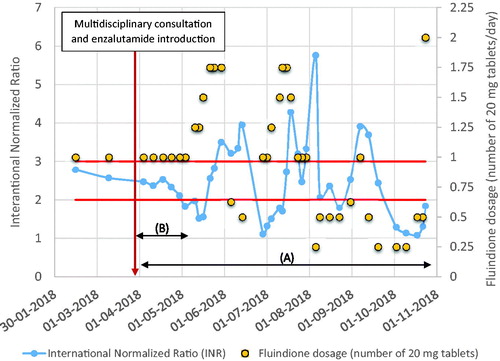
In late October 2018, the patient was admitted to the emergency department of a hospital near his home for hematemesis and rectorrhagia of medium and high severity. He had been hospitalized in June for the same symptoms of erosive gastritis and esophagitis upstream of a hiatal hernia. At this admission, treatment still included fluindione 20 mg (1/4 or 1/2 tablet according to INR) and the INR was at 5. The patient nevertheless reported a recent intake of 2 fluindione tablets at 2 days in a row because of infratherapeutic INR.
In total, a diagnosis of gastrointestinal bleeding on subcardiac fundal ulceration complicated by hemodynamic instability was retained in the context of VKA overdose. The patient received five packed red blood cells, and his hemoglobin level increased from 6.8 g/dL during hospitalization to 9.3 g/dL at the end of hospitalization.
The emergency department, in agreement with the patient’s referring cardiologist, finally initiated tinzaparin subcutaneously at an effective dose of 16,000 anti-Xa IU per day. This adaptation took place 8 months after the pharmacist recommendation at the primary oral chemotherapy consultations.
Discussion
In clinical use, enzalutamide is a strong CYP 3A4 inducer, as well as a moderate inducer of CYP 2C9 and CYP 2C19. Enzalutamide-induced reduction in the plasma concentrations of concomitant medications can be substantial, and especially lead to loss or reduction in the clinical anticoagulation effect of fluindione, which is metabolized up to 74% by CYP 2C9, resulting in a 50% decrease in its AUC (0.33–0.80) with enzalutamide [Citation2]. This ratio is consistent with the case of warfarin (same pharmacotherapeutic group as fluindione), with adjustments of 30–50% of the necessary doses [Citation3]. This drug interaction may lead to increased risk of venous thrombosis, considering the patient’s medical history of atrial fibrillation, the thromboembolic risk associated with tumor, and anticancer treatment. The enzyme-inducing mechanism of enzalutamide is clearly visible in . As mentioned in the summary of product characteristics, this enzyme-inducing effect occurred after approximately 1 month of treatment. In addition, we observed that the INR went beyond the limits fixed by the cardiologist and became less than 2 at the beginning of May, attesting the ineffectiveness of fluindione at a constant dosage of one tablet per day in ensuring thromboprophylaxis. Dose escalations of fluindione by 25%, 50%, up to 75% were performed in June. During the last 7 months, the INR was never stabilized and fluindione dosage fluctuated. Patient compliance was not known, but may play a role in this difficulty in stabilizing treatment.
Regarding therapeutic drug monitoring of digoxin, it appears that enzalutamide and its metabolites could falsely increase digoxinemia values [Citation4]. Follow-up of digoxinemia may not be advisable to ensure digoxin effectiveness in patients treated by both enzalutamide and digoxin.
The clinical signs of erosive gastritis and esophagitis were potentially the consequences of another drug interaction not previously mentioned. Indeed, pantoprazole is metabolized up to 80% by CYP 2C19; the enzyme-inducing effect of enzalutamide results in an AUC ratio of approximately 40% (0.24–0.67) [Citation2], an evidence of the inefficacy of the antacid treatment. This interaction was not thought to be relevant at the time of the first consultation because we usually try to terminate treatment with proton pomp inhibitors, such as pantoprazole, which are known to decrease the absorption of many concomitantly administered drugs, particularly oral chemotherapies. Given the patient’s history of hiatal hernia, the treatment was not interrupted.
This case raises the need for awareness, by the patient’s various interlocutors including his referring physicians, of the risk of major interactions, and for taking into account the whole medicinal care of a patient. In addition, these case points out the need for reliable tools that can quantitatively predict these interactions, which are complex, given the frequent polypharmacy in patients with prostate cancer [Citation5].
Clinical pharmacists play an important role in the evaluation and prevention of iatrogenic risks and drug interactions prior to the initiation of anticancer treatment, particularly for oral therapies. They can help oncologists and the patient's specialist doctors by playing a central role in the rationalization of treatments, including supportive care treatments and treatments for associated pathologies. They can propose the realization of therapeutic adaptations, neither to compromise the effectiveness of oncological treatment nor to destabilize long-term therapies.
In the particular case of enzalutamide prescription, cardiologists play an important role in reducing the cardiovascular risks associated with hormone therapy [Citation6], as well as preventing clinically relevant enzalutamide interaction with most drugs in this field.
In conclusion, drug–drug interaction analysis of the entire drug list is essential for patients who require treatment with enzalutamide and are receiving polypharmacy, including VKA, digoxin, or any substrate of CYP 3A4 or P-gp with narrow therapeutic window [Citation7].
Disclosure statement
The authors report no conflicts of interest. We declare no sources of funding for this report.
References
- Semenas J, Dizeyi N, Persson JL. Enzalutamide as a second generation antiandrogen for treatment of advanced prostate cancer. Drug Des Devel Ther. 2013;277:875–881.
- Estimation of the variation of exposure due to a drug-drug interaction - DDI-Predictor Academic version [Internet]. [cited 2018 Dec 27]. Available from: https://www.ddi-predictor.org/predictor/ddi
- Parrett JL, Reaves AB, Self TH, et al. Enzalutamide-warfarin interaction necessitating warfarin dosage adjustment: A case report of successful clinical management. J Clin Pharm Ther. 2018;43:276–279.
- Deguigne M, Brunet M, Abbara C, et al. Enzalutamide and analytical interferences in digoxin assays. Clin Toxicol. 2018;56:1150–1154.
- Sellers LE, Savas AN, Prentice M, et al. Prevalence of poly-pharmacy in the prostate cancer clinic and patient interest in a Medicine Review Service. J Oncol Pharm Pract. 2017;23:1–21.
- Veccia A, Maines F, Kinspergher S, et al. Cardiovascular toxicities of systemic treatments of prostate cancer. Nat Rev Urol. 2017;14:230.
- Gibbons JA, de Vries M, Krauwinkel W, et al. Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015;54:1057–1069.
