Abstract
Background: Prior reports have raised concerns that a prophylactic gastrostomy may be detrimental to long-term swallow function. This study evaluates patient-reported swallow function following chemoradiotherapy for oropharyngeal carcinoma in relation to the use of a prophylactic gastrostomy or nasogastric (NG) tube as required.
Material and methods: The MD Anderson Dysphagia Inventory (MDADI) was posted to 204 disease-free patients at least 2 years following chemoradiotherapy for oropharyngeal carcinoma between 2010 and 2014.
Results: Overall, 181/204 (89%) patients returned questionnaire at a median of 34 months post-treatment. 97/181 (54%) and 84/181 (46%) were managed with an approach of a prophylactic gastrostomy or NG tube as required, respectively. A prophylactic gastrostomy was associated with higher rates of enteral feeding (92% vs. 58%, p < .001), lower median percentage weight loss (7.0% vs. 9.4%, p < .001), increased duration of enteral feed (median 3.3 vs. 1.1 months, p < .001). There was no significant difference in patient-reported swallow function measured by MDADI summary scores and subscales for patients managed with an approach of prophylactic gastrostomy or NG as required. Duration of enteral feed correlated negatively with composite MDADI scores. A subgroup of 116/181 (64%) patients were documented as having been offered a choice of enteral feeding approach and therefore can be considered to represent clinical equipoise; there were no significant differences in MDADI scores according to route.
Conclusions: Despite concern regarding the use of a prophylactic gastrostomy in prior studies, the approaches of using a prophylactic gastrostomy or an NG tube as required to support patients during/after chemoradiotherapy for oropharyngeal carcinoma were associated with similar long-term swallow outcomes.
Introduction
Long-term dysphagia following chemoradiotherapy is a major treatment-related morbidity, and is strongly associated with health-related quality of life [Citation1,Citation2]. The majority of patients rate swallow function as a priority concern one year post-treatment [Citation1]. Patient- and tumour-related factors influencing swallow function include tumour size, baseline swallow function and age [Citation3,Citation4]; treatment-related factors include the use of concurrent chemotherapy, treatment technique and dose to pharyngeal constrictors [Citation4,Citation5].
Enteral feeding is commonly required to support nutrition during and soon after chemoradiotherapy. Rates of enteral feeding vary considerably between institutions, ranging between 50 and 100% [Citation6–9]. The route of enteral feeding is usually either a nasogastric (NG) tube inserted as required during/after treatment or a gastrostomy placed prophylactically prior to treatment (either a percutaneous endoscopic gastrostomy (PEG) or radiologically inserted gastrostomy (RIG)). Potential advantages of a prophylactic gastrostomy include lower rates of hospitalisation [Citation7,Citation10,Citation11], less weight loss [Citation10,Citation12–14], lower rates of tube blockage [Citation15], reduced rates of aspiration [Citation11], patient convenience/less impact on body image and superior quality of life soon after treatment [Citation13,Citation16,Citation17]. Advantages of a reactive NG tube approach include the avoidance of tube insertion in patients in whom enteral feeding support is not required, avoidance of morbidity associated with gastrostomy insertion [Citation18], shorter duration of enteral feeding [Citation7,Citation17] and reduced cost [Citation17]. Approaches to enteral feeding remains an area of highly variable practice [Citation19], with some centres routinely opting for a prophylactic gastrostomy [Citation20,Citation21] and others preferring the NG tube route [Citation8].
A critical issue in determining the optimal approach to enteral feeding is whether the timing and route influences long-term swallow function. Protracted enteral feeding may lead to deconditioning of the muscles involved in swallowing. A potential concern with insertion of a prophylactic gastrostomy is that the convenience of enteral feeding may lead to slower reintroduction of oral intake post-treatment [Citation15]. This is a highly controversial area, with some prospective [Citation17,Citation22] and retrospective [Citation15,Citation21,Citation23,Citation24] studies suggesting that approach of an NG tube as required may be associated with superior long-term swallow function. One randomised study did not find any difference in long-term swallow function (although lacked a validated dysphagia outcome) [Citation13] whilst two more recent randomised controlled trials have failed to complete recruitment [Citation17,Citation25]. A systematic review has recommended further research in this area of variable practice [Citation26].
In our centre, we have used an approach of either a prophylactic gastrostomy or NG tube as required based, commonly offering a choice to patients. The aim of this study is to evaluate long term patient reported swallow outcomes evaluated using the MD Anderson Dysphagia Inventory (MDADI) at least 2 years following completion of chemoradiotherapy for oropharyngeal carcinoma in relation to the chosen approach to enteral nutrition. In order to limit potential confounding factors a subgroup of patients offered a choice of tube route is analysed in addition to the cohort as a whole.
Methods
Study design
The study was approved by the Institutional Quality Improvement Board. All patients were treated at Leeds Cancer Cancer within the public healthcare sector. This practice is based upon a hub-and-spoke model with patients seen initially in surrounding cancer units (Bradford, Pinderfields, York) or in Leeds, with pre- and post-treatment follow up and support delivered locally in these units.
Consecutive patients with locally advanced oropharyngeal carcinoma treated with concurrent chemoradiotherapy were identified from electronic records treated between October 2010 and December 2014. Inclusion criteria were oropharyngeal primary, squamous cell pathology, stage III/IV, definitive non-surgical treatment delivered with curative intent, use of intensity modulated radiotherapy (IMRT), radiotherapy target including bilateral neck, concurrent platinum chemotherapy, no prior therapeutic surgery, disease free at time of study (at least 2 years from final day of radiotherapy). Exclusion criteria were therapeutic enteral feeding commenced prior to radiotherapy, treatment given for recurrent disease or second primary tumour, radiotherapy to the unilateral neck only. Ten patients with oropharyngeal carcinoma who had required therapeutic enteral feeding prior to treatment were excluded from the study (did not receive a questionnaire).
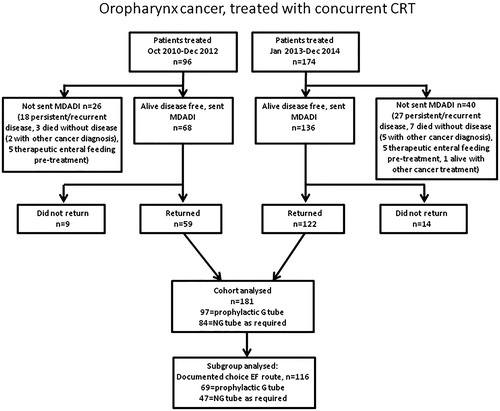
Patients included in the study who had completed treatment ≥2 years previously were posted an explanatory letter inviting them to complete and return an enclosed copy of the MDADI questionnaire. In the event of no response, a further follow up letter and another copy of the questionnaire was sent after approximately a one month interval. The initial cohort treated between October 2010 and December 2012 had completed the postal MDADI questionnaire in January 2015 (all >2 years post treatment) as previously reported [Citation23] were included within this analysis (59/68 (87%) patients returned questionnaires). The MDADI was posted in January 2017 to patients treated between January 2013 and December 2014 (122/136 (90%) patients returned questionnaires). Overall questionnaires were returned by 181/204 (89%) patients.
The MDADI is a validated reliable self-administered questionnaire which uses 20 questions designed to assess patients’ perception of their swallowing ability and how this may impact upon their quality of life [Citation27,Citation28]. Each individual question is scored on a 5-point scale with responses ranging from ‘strongly agree’ to ‘strongly disagree’. Questions are divided into emotional, functional and physical subscales. Two summary scores are obtained; the global scale is a single question scored individually and the composite is a score summarising the remaining 19 questions. Summary and subscale scores are normalised to a range from 20 to 100. Higher scores indicate superior swallow-related quality of life/function.
Pretreatment diet was categorised using prospectively collected pretreatment dietetic and nursing assessments into nil by mouth, sips, pureed, soft, normal.
Pre- and post-treatment support
All patients routinely underwent dietetic, nursing and speech and language therapy (SLT) assessments pretreatment. During treatment, patients were routinely reviewed twice weekly by medical and nursing teams with additional dietetic and SLT input as required. Post-treatment rehabilitation was offered to all patients as part of standard care within the public health care service in dedicated combined nursing, dietetic and SLT post-treatment clinics.
Approach to enteral feeding support
During this time the approach at Leeds Cancer Centre to the use of either a prophylactic or reactive approach to enteral feeding was to consider either a prophylactic gastrostomy or reactive NG tube depending upon clinician and patient preference. In general, if the treating oncologist considered either route of feeding to be appropriate our practice was for the treating clinician to discuss options with regard to enteral feeding with the patient at initial consultation with subsequent discussions following between specialist dieticians/nurses and the patient. If, based upon their assessment of patient/social/tumour/treatment factors the clinician considered a particular route of enteral feeding support preferable a choice may not have been offered. For the purposes of this study, electronic medical notes and dietetic records were retrospectively reviewed to determine if there was documentation of patients being offered a choice of approach to enteral feeding or whether there was a clinical decision to recommend a particular approach to nutritional support.
During and after treatment decisions to commence use of a gastrostomy to support nutrition (in the prophylactic gastrostomy group) or to insert of an NG tube (in the NG tube as required group) were based upon reviews by the treating clinician and dietetic team in discussion with patients. These decisions were made based upon clinical judgment involving multiple factors including assessment of difficulties maintaining nutrition, weight loss, how much treatment left to complete, potential for maintaining oral nutrition with analgesia, patient wishes. We do not use institutional protocols pre-determining requirements for enteral feeding, for example based upon pre-defined weight loss cutoffs.
Treatment details
All patients in this era of treatment (October 2010 to December 2014) were treated according to the same institutional protocols with IMRT.
Induction chemotherapy
Induction chemotherapy (ICT) in this era was delivered to selected patients based upon clinician preference, patients and disease factors. ICT constituted either TPF (docetaxel 75 mg/m2, cisplatin 75 mg/m2, 5-flurouracil (5-FU) 750 mg/m2 days 2–5) [Citation29] or PF (cisplatin 80 mg/m2 and 5-FU 800 mg/m2) [Citation30].
Concurrent chemotherapy
Standard concurrent chemotherapy was cisplatin 100 mg/m2 days 1 and 29. Carboplatin AUC4 was substituted in the event of a contraindication to cisplatin.
Radiotherapy
IMRT was used to treat all patients in this analysis and was delivered as previously described [Citation23,Citation31].
A compartmental approach to target volume delineation was used during this era of 20102014, in line with the approach in the PARSPORT study [Citation32], and as previously described [Citation23,Citation31]. Primary tumour CTV included the GTV plus 10 mm and the anatomical compartment i.e., the whole oropharynx, edited to anatomical boundaries to exclude air and/or bone without evidence of invasion. The high-dose nodal CTV included the whole involved nodal level. Radiologically uninvolved nodal levels were treated at an intermediate or lower dose level according to clinician preference. Retropharyngeal lymph nodes were routinely included in cases with positive level II lymph nodes and/or posterior pharyngeal wall involvement. A PTV was created by an isotropic expansion of 4 mm. The standard dose was 70 Gy in 35 fractions over 7 weeks. The dose to the elective target volume was 57 Gy in 35 fractions with an intermediate dose level of 63 Gy in 35 fractions used at clinician discretion. Treatment was delivered using a 5–7 angle step and shoot IMRT technique.
Statistical analysis
Comparison of patient, tumour and treatment details between groups (i.e., prophylactic gastrostomy or reactive NG) was performed with a Mann–Whitney U or chi-squared test as appropriate to test for differences in subgroups analysed. MDADI scores between groups were also compared using the Mann–Whitney U test; for the purposes of analysis T stage was analysed as T1/2 vs. T3/4, nodal stage analysed as N0/1 vs. N2/3, grade 1/2 vs. 3. Duration of enteral feed was calculated from time from final fraction of radiotherapy to cessation of enteral feed (not tube removal). Spearman’s rank correlation coefficient (rho) was used to evaluate the association between composite MDADI scores and continuous variables and ordinal clinical variables with more than two groups (age, number of concurrent chemotherapy cycles, pretreatment diet, pretreatment body mass index (BMI), post-treatment diet, % weight loss during treatment, duration of enteral feed post-treatment. For non-ordinal data and ordinal data with only two groups the Mann–Whitney U test (two groups) and Kruskall-- Wallace tests (>2 groups) were used to investigate relationships between clinical factors and composite MDADI scores (gender, subsite, T stage, N stage, grade, prophylactic gastrostomy or reactive NG, smoking status, alcohol intake, use of ICT and use of EF). Statistical significance was declared at p < .05.
Results
Whole cohort (n = 181)
Overall, 204/270 (76%) patients treated with chemoradiotherapy for oropharyngeal carcinoma were sent the MDADI questionnaire. Reasons for not sending questionnaires to 66 patients are shown in . Ten patients who required therapeutic enteral feeding pretreatment were not sent a questionnaire. Excluding 10 patients requiring therapeutic enteral feed pretreatment, 138/260 (53%) patients in the overall cohort were managed with a prophylactic gastrostomy; this included 29/56 (52%) patients not sent a questionnaire.
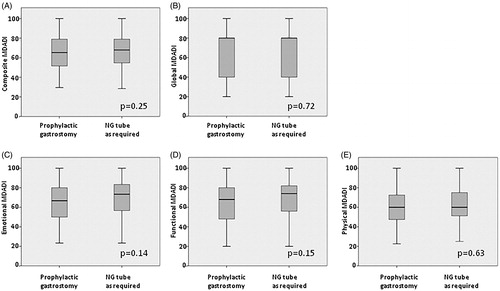
Figure 2. Box plots illustrating MDADI summary and subscale scores for whole cohort (n = 181) comparing patients managed with prophylactic gastrostomy (n = 97) or NG as required (n = 84). (A) Composite, (B) Global, (C) Emotional, (D) Functional and (E) Physical scales.
181/204 (89%) patients returned questionnaires at a median of 34 months (range 24–59) post treatment. All were disease free at the time of questionnaire completion and had not required enteral feeding pretreatment. For the initial cohort of 59 patients treated between October 2010 and December 2012 median time from end of treatment to questionnaire completion was 35 months (range 24–59). For the subsequent cohort of 122 patients treated between January 2013 and December 2014 median time from end of treatment to questionnaire completion was 34 months (range 24–48). 97/181 (54%) had been managed with a prophylactic gastrostomy and 84/181 (46%) with an approach of NG tube as required. Median time from end of treatment to questionnaire completion for the prophylactic gastrostomy group was 34 months (24–59) and for the NG tube as required group 33 months (24–59). Baseline patient, tumour and treatment characteristics for the whole group and these two subgroups are summarised in . Although a small group, characteristics and routes of enteral feeding of the 23 patients who did not return the questionnaire appear to be similar to the overall cohort (Supplementary Table 1). 12/23 (%) patients who did not return a questionnaire had been managed with a prophylactic gastrostomy. Of the 181 patients returning the questionnaire, those managed with a prophylactic gastrostomy were significantly more likely to have more advanced T stage (p < .029), received ICT (22% vs. 3%, p < .001); a higher proportion of patients in the prophylactic gastrostomy group were receiving a pureed or soft diet pretreatment (21% vs. 8%) although the difference was not significant. Of the 84 patients, 49 were managed with the approach of NG feeding as required ended up receiving NG feeding. Four of the remaining 35 patients who did not undergo NG feeding were documented to have been recommended to commence NG feeding but declined. Prophylactic gastrostomy was associated with lower median percentage weight loss (7% vs. 9.4%, p < .001), more likely to receive enteral tube feeding (92% vs. 58%, p < .001), increased median duration of enteral feeding post-treatment (median 3.3 vs. 1.3 months, p < .001).
Table 1. Patient, tumour and treatment details in whole cohort (n = 181).
and show a comparison of MDADI composite and global summary scores, along with the emotional, functional and physical subscales. Median composite MDADI score for the whole group was 66.3, with no significant difference between the prophylactic gastrostomy group and NG tube as required groups (median 65.3 and 67.9 respectively, p = .25). There were no significant differences for the global score or subscales for the two groups.
Table 2. MDADI scores according to intended enteral feeding route (n = 181).
Subgroup offered a choice of prophylactic gastrostomy or NG tube as required (n = 116)
The choice of enteral feeding strategy can be considered to be in equipoise for patients offered a choice by the clinical team. On retrospective review of records, there was documentation of a choice being offered to 116/181 (64%) patients. For the remaining 65/181 (36%) of patients, the clinician made a recommendation to 49/65 patients rather than offering a choice, there was no documentation of whether a choice was offered/why route of enteral feeding chosen in 11/65 patients, and 5/65 patients made a decision regarding route of enteral feeding without there being a documented choice offered. Of these 65 patients, 28 were without a documented choice underwent a prophylactic gastrostomy.
In order to limit potential imbalances between the two groups analysed in the overall cohort, the subgroup of 116/181 (64%) of patients documented to have been offered a choice of prophylactic gastrostomy or NG tube as required were analysed. 69/116 (59%) and 47/116 (41%) were managed with prophylactic gastrostomy or NG tube as required. Median follow up for the prophylactic gastrostomy group was 35 months (24–59) and for the NG tube as required group was 33 months (24–59). Patient, tumour and treatment details are summarised in Supplementary Table 2. The group of patients managed with a prophylactic gastrostomy had significantly greater rates of heavy alcohol intake (23% vs. 6%, p = .031). There was no significant difference in pre- or post-treatment oral intake. The use of enteral feed was more likely in the prophylactic gastrostomy group (90% vs. 57%, p < .001) and median duration of enteral feed was longer (3.3 vs. 1.3 months, p < .001). Supplementary Table 3 shows that within this group of patients offered a choice of prophylactic gastrostomy or NG tube as required there was no significant difference in summary or subscale MDADI scores; median composite scores were 65.8 vs. 67.9 (p = .42), respectively.
Associations between clinical factors and composite MDADI scores
shows the analysis of associations between patient, tumour and treatment factors and the composite MDADI score. Abnormal pretreatment diet was associated with a lower composite MDADI score in the whole cohort. Duration of enteral feed post-treatment showed a weak but significant negative correlation with composite MDADI scores in both the whole cohort and subgroup offered the choice; route of enteral feeding showed no association with MDADI composite score. Use of enteral feeding was associated with lower composite scores in the whole group. Current smokers had significantly lower composite scores compared to never and ex-smokers in the whole cohort and in the subgroup offered choice of tube.
Table 3. Correlation between clinical factors and composite MDADI score.
Discussion
Previous studies have provided conflicting results with regard to the impact of route of enteral tube feeding upon swallow outcomes. One randomised study of 134 patients comparing a prophylactic gastrostomy with a reactive approach has been completed [Citation13,Citation33]; no significant differences were noted in swallow function were noted as measured by the EORTC-QLQ-H&N35 questionnaire, oral intake scale, oesophageal interventions or tube dependence. Limitations of this study include heterogeneous treatments/tumour sites and the lack of a validated dysphagia scale [Citation33]. The recent TUBE trial was closed following poor recruitment [Citation25]. A prior randomised study was converted to a prospective study after poor accrual [Citation17]; in this study, an analysis of 32 PEG and 73 NG patients grade 3 dysphagia was found to significantly higher 6 months post treatment for PEG patients. A recent prospective cohort study [Citation22] of 53 oropharyngeal cancer patients with normal pretreatment diet found higher MDADI scores at 12 months post treatment for patients managed with a reactive NG tube vs. a prophylactic gastrostomy (mean total MDADI 72.3 vs. 61.2) although differences were not statistically significant. In a retrospective analysis of 120 patients treated with chemoradiotherapy for head and neck cancer [Citation21], gastrostomy dependence was higher at 12 months following a prophylactic gastrostomy (21% vs. 0%]. A small retrospective matched pair analysis of 31 patients following chemoradiotherapy showed significantly inferior MDADI scores >2 years post treatment for the group managed with a prophylactic gastrostomy [Citation24]. Ward et al. [Citation15] analysed long-term swallow outcomes (defined as need for oesophageal dilatation/hospitalisation for aspiration pneumonia/further use of feeding tube after initial tube removed) in 78 patients who had required enteral tube feeding support during chemoradiotherapy; the 5 year incidence of severe late dysphagia was 30.8% in the reactive NG group (n = 36) vs. 56% in a reactive PEG group (n = 17) and 60.9% in a prophylactic PEG group (n = 25). By contrast, a report of series of patients managed with a gastrostomy showed low rates of long-term tube dependence and favourable long-term swallow outcomes for the majority of patients [Citation20].
This study seeks to explore the critical clinical question of whether a prophylactic gastrostomy or NG tube as required has superior outcomes in terms of long-term swallow recovery. The assessment of dysphagia is complex with a battery of differing tools, including physician assessed toxicity scores, physical outcomes including videofluoroscopy and multiple patient reported measures [Citation3]. It has been observed that physician-measured and patient-reported assessment tools do not necessarily correlate, with data suggesting that patient-reported measures may rate dysphagia more severely [Citation34–36]. Patient-reported outcome measures are therefore key to assessment of swallow function. The MDADI is a widely employed, reliable and validated patient reported outcome measure [Citation27,Citation28]. Recovery of swallow function is recognised to be a gradual process post-chemoradiotherapy; for example MDADI scores have been shown to steadily improve up to an assessment 12 months post-treatment and may have not plateaued by that timepoint [Citation1]. In a similar manner, salivary flow has been shown to improve until at least 2 years post-treatment [Citation32]. In this study, a timepoint of at least 2 years post-treatment was chosen to reflect long-term outcomes.
Analysis of the whole cohort of 181 patients shows that there was no significant difference in summary and subscale MDADI scores between the prophylactic gastrostomy and NG tube as required groups of patients. This is despite an imbalance in the baseline characteristics between these two groups with significantly higher T stage and increased use of ICT and a non-significant trend towards higher frequency of pretreatment soft or pureed diet in the group of patients managed with a prophylactic gastrostomy. This implies the likelihood of patients with bulkier disease/poorer baseline swallow function being managed with a prophylactic gastrostomy. We have previously performed a combined analysis of long term patient reported swallow function using the MDADI in 207 patients with the earlier part of this cohort combined with that from another large UK center (The Christie Hospital, Manchester) [Citation37]. Our centres had a contrasting approach to enteral feeding, with The Christie Hospital not using prophylactic gastrostomy tubes. Although there are potential confounding factors, it is interesting to note that despite these differing strategies there was no difference in MDADI-measured swallow outcomes between the centres.
Previous studies are limited by potential confounding factors affecting why patients had received a prophylactic gastrostomy rather than a reactive approach to feeding [Citation17,Citation21–24,Citation38]. Clinicians may be more likely to recommend a prophylactic gastrostomy tube to patients they consider at higher risk of requiring enteral feeding support i.e., in patients with more advanced/bulky disease. It is interesting to note that within the group of patients offered a choice of approach, a higher proportion of patients with a history of heavy alcohol intake opted for a prophylactic gastrostomy. The reasons for this are unclear, with potential confounding factors such as social status and influence of the way in which the choice is put by the clinicians which cannot be elucidated on this analysis. A propensity to favour a prophylactic gastrostomy in patients who may be at risk of poorer long term swallow outcomes (e.g., due disease factors) may at least partly explain why use of a prophylactic gastrostomy has been associated with poorer long term swallow function [Citation21,Citation22,Citation24,Citation39]. The greater likelihood of patients with more advanced T stage being managed with a prophylactic gastrostomy was observed in our whole cohort. We considered that greater clinical equipoise in choosing tube route was likely for the 116 patients for whom there was a documented choice of approach to enteral feeding. In this well-balanced subgroup, there were no differences in MDADI scores according to the chosen enteral feeding approach.
Previous data have suggested that a period of >2 weeks nil by mouth during treatment is detrimental to swallow function [Citation40]. The data presented here demonstrate that an increased duration of enteral feeding correlates with poorer patient-reported long-term swallow function. Although we do not have data regarding duration of any periods in which patients were nil by mouth per se, and there are potential confounding factors, this finding does support a hypothesis that periods of limited oral intake may predispose to inferior swallow recovery. Based upon these observations, it seems reasonable to suggest that swallow rehabilitation soon after treatment should be a high priority. The association between current smoking and long-term swallow function also highlights the importance of stopping smoking prior to treatment.
The data in the overall cohort demonstrated that patients managed with a prophylactic gastrostomy on average lose significantly less weight than patients managed with the NG as required approach; this is despite no significant difference in radiotherapy dose/delivery of cycles of concurrent chemotherapy. These observations are in line with prior series by ourselves [Citation7] and others [Citation10,Citation12–14,Citation17]. This finding did not reach significance in the subgroup offered a choice although the same magnitude of difference in percentage weight loss was observed. The reason for the reduced weight loss with a prophylactic gastrostomy is likely to be related to the observation that a significantly higher proportion of these patients receiving enteral feeding (92% vs. 58%, p < .001 and that median duration of feeding is significantly longer (median 3.3 vs. 1.2 months, p < .001). Overall patients with a gastrostomy already in situ are more likely to be prepared to commence enteral feeding support and to be prepared to continue enteral feeding for longer with a tube which is not visible to others. Related to this are observations from prior studies [Citation13,Citation16,Citation17] that quality of life on completion of treatment is higher in patients managed with a gastrostomy.
There are several limitations to this study to consider. These include the absence of data on human papilloma virus for a sufficient part of the study for useful analysis. However, since proportions of current and ex-smokers are similar between the groups it seems likely that HPV prevalence is similar. Pretreatment MDADI data are not available to assess for any differences in baseline swallow function between the groups. However, there was no significant difference in documented diet consistency between groups. The approach to target delineation in patients treated in this era is now historical. Current recommendations to CTV delineation are highly volumetric [Citation41]; it is likely that this approach may impact favourably upon dysphagia outcomes with current interest in dysphagia-sparing IMRT [Citation42]. The approach of evaluating swallow function >2 years post treatment is designed to allow assessment of ‘stable’ dysphagia. There is the possibility that patients with more severe dysphagia may not survive until the 2 year timepoint influencing findings; however, as shown in , only three patients died prior to this timepoint without evidence of disease recurrence or another cancer diagnosis.
In summary, these data strongly suggest that the selection of a prophylactic gastrostomy compared with an NG tube as required approach does not negatively impact upon long-term swallow function.
Supplemental Material
Download MS Word (32.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Roe JW, Drinnan MJ, Carding PN, et al. Patient-reported outcomes following parotid-sparing intensity-modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncol. 2014;50:1182–1187.
- Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg. 2011;145:767–771.
- Batth SS, Caudell JJ, Chen AM. Practical considerations in reducing swallowing dysfunction following concurrent chemoradiotherapy with intensity-modulated radiotherapy for head and neck cancer. Head Neck. 2014;36:291–298.
- Frowen J, Cotton S, Corry J, et al. Impact of demographics, tumor characteristics, and treatment factors on swallowing after (chemo)radiotherapy for head and neck cancer. Head Neck. 2010;32:513–528.
- Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;73:410–415.
- Koyfman SA, Adelstein DJ. Enteral feeding tubes in patients undergoing definitive chemoradiation therapy for head-and-neck cancer: a critical review. Int J Radiat Oncol Biol Phys. 2012;84:581–589.
- Williams GF, Teo MT, Sen M, et al. Enteral feeding outcomes after chemoradiotherapy for oropharynx cancer: a role for a prophylactic gastrostomy? Oral Oncol. 2012;48:434–440.
- Clavel S, Fortin B, Despres P, et al. Enteral feeding during chemoradiotherapy for advanced head-and-neck cancer: a single-institution experience using a reactive approach. Int J Radiat Oncol Biol Phys. 2011;79:763–769.
- Nguyen NP, North D, Smith HJ, et al. Safety and effectiveness of prophylactic gastrostomy tubes for head and neck cancer patients undergoing chemoradiation. Surg Oncol. 2006;15:199–203.
- Piquet MA, Ozsahin M, Larpin I, et al. Early nutritional intervention in oropharyngeal cancer patients undergoing radiotherapy. Support Care Cancer. 2002;10:502–504.
- Hughes BG, Jain VK, Brown T, et al. Decreased hospital stay and significant cost savings after routine use of prophylactic gastrostomy for high-risk patients with head and neck cancer receiving chemoradiotherapy at a tertiary cancer institution. Head Neck. 2013;35:436–442.
- Lee H, Havrila C, Bravo V, et al. Effect of oral nutritional supplementation on weight loss and percutaneous endoscopic gastrostomy tube rates in patients treated with radiotherapy for oropharyngeal carcinoma. Support Care Cancer. 2008;16:285–289.
- Silander E, Nyman J, Bove M, et al. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head Neck. 2012;34:1–9.
- Romesser PB, Romanyshyn JC, Schupak KD, et al. Percutaneous endoscopic gastrostomy in oropharyngeal cancer patients treated with intensity-modulated radiotherapy with concurrent chemotherapy. Cancer. 2012;118:6072–6078.
- Ward MC, Bhateja P, Nwizu T, et al. Impact of feeding tube choice on severe late dysphagia after definitive chemoradiotherapy for human papillomavirus-negative head and neck cancer. Head Neck. 2016;38:E1054–E1060.
- Salas S, Baumstarck-Barrau K, Alfonsi M, et al. Impact of the prophylactic gastrostomy for unresectable squamous cell head and neck carcinomas treated with radio-chemotherapy on quality of life: prospective randomized trial. Radiother Oncol. 2009;93:503–509.
- Corry J, Poon W, McPhee N, et al. Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo)radiation. Head Neck. 2009;31:867–876.
- Dharmarajan TS, Unnikrishnan D. Tube feeding in the elderly. The technique, complications, and outcome. Postgrad Med. 2004;115:51–54.
- Moor JW, Patterson J, Kelly C, et al. Prophylactic gastrostomy before chemoradiation in advanced head and neck cancer: a multiprofessional web-based survey to identify current practice and to analyse decision making. Clin Oncol (R Coll Radiol). 2010;22:192–198.
- Crombie JM, Ng S, Spurgin AL, et al. Swallowing outcomes and PEG dependence in head and neck cancer patients receiving definitive or adjuvant radiotherapy +/- chemotherapy with a proactive PEG: a prospective study with long term follow up. Oral Oncol. 2015;51:622–628.
- Chen AM, Li BQ, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78:1026–1032.
- Goff D, Coward S, Fitzgerald A, et al. Swallowing outcomes for patients with oropharyngeal squamous cell carcinoma treated with primary (chemo)radiation therapy receiving either prophylactic gastrostomy or reactive nasogastric tube: a prospective cohort study. Clin Otolaryngol. 2017;42:1135–1140.
- Sethugavalar B, Teo MT, Buchan C, et al. Impact of prophylactic gastrostomy or reactive NG tube upon patient-reported long term swallow function following chemoradiotherapy for oropharyngeal carcinoma: a matched pair analysis. Oral Oncol. 2016;59:80–85.
- Oozeer NB, Corsar K, Glore RJ, et al. The impact of enteral feeding route on patient-reported long term swallowing outcome after chemoradiation for head and neck cancer. Oral Oncol. 2011;47:980–983.
- Paleri V, Patterson J, Rousseau N, et al. Gastrostomy versus nasogastric tube feeding for chemoradiation patients with head and neck cancer: the TUBE pilot RCT. Health Technol Assess. 2018;22:1–144.
- Shaw SM, Flowers H, O’Sullivan B, et al. The effect of prophylactic percutaneous endoscopic gastrostomy (PEG) tube placement on swallowing and swallow-related outcomes in patients undergoing radiotherapy for head and neck cancer: a systematic review. Dysphagia. 2015;30:152–175.
- Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870–876.
- Hutcheson KA, Barrow MP, Lisec A, et al. What is a clinically relevant difference in MDADI scores between groups of head and neck cancer patients? Laryngoscope. 2016;126:1108–1113.
- Prestwich RJ, Oksuz DC, Dyker K, et al. Feasibility and efficacy of induction docetaxel, cisplatin, and 5-fluorouracil chemotherapy combined with cisplatin concurrent chemoradiotherapy for nonmetastatic stage IV head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2011;81:e237–e243.
- Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
- Bayman E, Prestwich RJ, Speight R, et al. Patterns of failure after intensity-modulated radiotherapy in head and neck squamous cell carcinoma using compartmental clinical target volume delineation. Clin Oncol (R Coll Radiol). 2014;26:636–642.
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136.
- Axelsson L, Silander E, Nyman J, et al. Effect of prophylactic percutaneous endoscopic gastrostomy tube on swallowing in advanced head and neck cancer: a randomized controlled study. Head Neck. 2017;39:908–915.
- Frowen J, Drosdowsky A, Perry A, et al. Long-term swallowing after chemoradiotherapy: prospective study of functional and patient-reported changes over time. Head Neck. 2016;38:E307–E315.
- Jensen K, Lambertsen K, Torkov P, et al. Patient assessed symptoms are poor predictors of objective findings. Results from a cross sectional study in patients treated with radiotherapy for pharyngeal cancer. Acta Oncol. 2007;46:1159–1168.
- Frowen JJ, Perry AR. Swallowing outcomes after radiotherapy for head and neck cancer: a systematic review. Head Neck. 2006;28:932–944.
- Dixon L, Ramasamy S, Cardale K, et al. Long term patient reported swallowing function following chemoradiotherapy for oropharyngeal carcinoma. Radiother Oncol. 2018;128:452–458.
- Prestwich RJ, Teo MT, Gilbert A, et al. Long-term swallow function after chemoradiotherapy for oropharyngeal cancer: the influence of a prophylactic gastrostomy or reactive nasogastric tube. Clin Oncol (R Coll Radiol). 2014;26:103–109.
- Corry J. Feeding tubes and dysphagia: cause or effect in head and neck cancer patients. J Med Imaging Radiat Oncol. 2009;53:431–432.
- Gillespie MB, Brodsky MB, Day TA, et al. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114:1362–1367.
- Gregoire V, Evans M, Le QT, et al. Delineation of the primary tumour clinical target volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol. 2018;126:3–24.
- Petkar I, Rooney K, Roe JW, et al. DARS: a phase III randomised multicentre study of dysphagia- optimised intensity- modulated radiotherapy (Do-IMRT) versus standard intensity- modulated radiotherapy (S-IMRT) in head and neck cancer. BMC Cancer. 2016;16:770.