Abstract
Background: Due to long-standing concerns that heavy-load lifting could increase the risk of developing lymphedema, breast cancer survivors have been advised to refrain from resistance exercise with heavy loads. This study prospectively evaluated the effect of heavy-load resistance exercise on lymphedema development in women receiving chemotherapy for breast cancer.
Material and Methods: Physically inactive women receiving adjuvant chemotherapy for breast cancer (n = 153) were randomized to a HIGH (supervised, multimodal exercise including heavy-load resistance exercise: 85–90% 1 repetition maximum [RM], three sets of 5–8 repetitions) versus LOW (pedometer and one-on-one consultations) 12-week intervention. Outcomes (baseline, 12 and 39 weeks) included lymphedema status (extracellular fluid [bioimpedance spectroscopy] and inter-arm volume % difference [dual-energy X-ray absorptiometry], lymphedema symptoms [numeric rating scale 0–10]), upper-extremity strength (1 RM), and quality of life domains (EORTC- BR23). Linear mixed models were used to evaluate equivalence between groups for lymphedema outcomes (equivalence margins for L-Dex, % difference and symptoms scale: ±5, ±3% and ±1, respectively). Superiority analysis was conducted for muscle strength and quality of life domains.
Results: Postintervention equivalence between groups was found for extracellular fluid (0.4; 90% CI −2.5 to 3.2) and symptoms of heaviness (−0.2; −0.6 to 0.2), tightness (−0.1; −0.8 to 0.6) and swelling (0.2; −0.4 to 0.8). Nonequivalence was found for inter-arm volume % difference (−3.5%; −17.3 to 10.3) and pain (−0.7; −1.3 to 0), favoring HIGH. Strength gains were superior in the HIGH versus LOW group (3 kg; 1 to 5, p < .05). Further, clinically relevant reductions in breast (−11; −15 to −7) and arm (−6; −10 to −1) symptoms were found in the HIGH group.
Conclusion: Findings suggest that physically inactive breast cancer survivors can benefit from supervised heavy-load resistance exercise during chemotherapy without increasing lymphedema risk.
Trial registration: ISRCTN13816000
Introduction
Breast cancer-related arm lymphedema (BCRL) is a chronic condition characterized by swelling of the arm on the surgical side, experienced by almost a quarter of breast cancer survivors and has adverse physical, social and psychological ramifications [Citation1–4]. While the pathophysiology of BCRL remains unclear, consistent evidence supports several risk factors including more extensive surgery (mastectomy and axillary lymph node dissection and greater number of lymph nodes removed), receipt of adjuvant therapies (chemotherapy and radiotherapy) and lifestyle-related factors such as obesity and physical inactivity [Citation1].
Receipt of adjuvant chemotherapy for breast cancer is associated with declines in physical activity [Citation5]. This in turn contributes to common increases in body weight [Citation6–9] characterized by no change or decline in muscle mass in the presence of increased body fat (sarcopenic obesity) [Citation6] and is adversely associated with reductions in muscle strength and functional impairment [Citation10,Citation11]. Therefore, interventions that thwart sarcopenic obesity are pertinent, with heavy-load resistance-exercise considered an effective strategy. This is due to the dose–response relationship, whereby heavier loads have been shown to elicit greater gains in muscular size, structure and function than with lower loads [Citation12,Citation13].
However, there are anecdotal concerns that resistance exercise with heavy loads may trigger the development of BCRL [Citation14,Citation15]. While previous interventions have demonstrated the safety of resistance exercise of low-to-moderate loads (60–80% of 1 repetition maximum (RM) or 8-20 RM) [Citation14,Citation16–19], no studies to date have prospectively assessed repeated exposure to resistance exercise with heavy loads.
Therefore, we prospectively compared the effect of a supervised, multimodal intervention including heavy-load resistance exercise (80–90% 1 RM or 5–8 RM) with a home-based individual walking intervention in physically inactive breast cancer survivors. A full report detailing the results on aerobic capacity (the primary outcome), body composition and quality of life can be viewed elsewhere (ISRCTN13816000, Møller T et al., in submission). The purpose of this manuscript is to report on the effect of the interventions on BCRL and upper-extremity outcomes (secondary outcomes). We hypothesized that changes in BCRL outcomes would be similar irrespective of intervention allocation. Further, we hypothesized that participation in the multimodal intervention would yield significant increases in upper-extremity muscular strength and breast cancer-specific functional and symptom domains compared to the walking intervention.
Material and methods
This study utilized a parallel group, randomized design (n = 153; detailed description of study design and methods have been previously reported) [Citation20,Citation21]. The study was conducted at the University Hospitals Centre for Health Research, Copenhagen University Hospital, Rigshospitalet. Written informed consent was provided by all participants before inclusion to the study. The study was approved by the Danish Capital Regional Ethics Committee (H-1-2011-131) and the Danish Data Protection Agency (2011-41-6349) and registered at Current Controlled Trials (ISRCTN13816000).
Participants
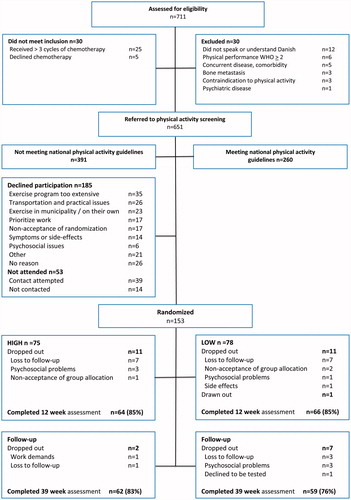
Women referred to adjuvant chemotherapy for stage I–III breast cancer at the oncology departments of The Copenhagen University Hospital, Rigshospitalet (RH) and Herlev Hospital (HE) were screened for eligibility by nurses/physicians upon initiation of chemotherapy. Women were eligible if they had a World Health Organization performance status 0–1, and retrospectively rated their physical activity levels three months prior to diagnosis as less than 150 minutes of, moderate- intensity physical activity and/or 2 × 20 minutes of high-intensity exercise per week [Citation22]. If initial criteria were met, women were then referred to the research team and matched against exclusion criteria [Citation20,Citation21] ().
Randomization
After successful completion of all baseline assessments (6–9 weeks postsurgery), women were sequentially numbered and stratified by age (<48/48+ years) and hospital (RH/HE). Intervention allocation (1:1) was determined by a computerized, random number generated at the Copenhagen Trial Unit.
Guidelines regarding BCRL
Participants were asked if they had received treatment for BCRL at the baseline assessment (either preventatively or as a means to reduce swelling after a diagnosis of BCRL) and were given verbal and written information, highlighting current evidence-based risk factors for developing BCRL [Citation1]. Women diagnosed with lymphedema were not excluded. Participants were encouraged to contact study personnel if signs or symptoms of BCRL development presented or exacerbation of an existing BCRL occurred during the study period. Either scenario would then instigate referral to a lymphedema therapist for evaluation and treatment as well as individual modifications of the exercise interventions based on response to prescribed intensities and modalities.
Interventions
High
Participants randomized to the HIGH group participated in a supervised twelve-week, group-based exercise program. The first six weeks consisted of a previously described exercise program ‘Body and Cancer’ [Citation20,Citation23,Citation24], which entailed multimodal sessions including low- and high-intensity components. The following six weeks consisted of an ‘All sport’ exercise program focusing on high-intensity components combined with other aerobic activities performed at moderate to high intensities. (Supplementary Table 1) [Citation20]. The high-intensity component included an aerobic-based warm-up followed by resistance exercise and 15–30 minutes of cardiovascular interval training on stationary bikes with peak loads equivalent to 85–95% maximal heart rate. The resistance exercise program comprised of six machine-based exercises (Technogym®, Gamettola, Italy) targeting major muscle groups of the body including the chest press and latissimus pull down. Resistance exercise loads were based on a 1 RM strength test performed during the first exercise session. During the first week, participants were instructed to lift loads corresponding to 2–3 sets of 8–12 repetitions at 70% 1 RM, progressing to 80% 1 RM in week two. From week three forward, loads lifted corresponded to 3 sets of 5–8 repetitions at 80–90% 1 RM. To ensure progression, resistance exercise programs were adjusted every third week based on new 1 RM testing [Citation20,Citation23].
Low
The LOW intervention involved an individualized, home-based walking program supported by a pedometer and one-on-one consultations (Supplementary Table 1). Participants were issued an Omron Walking Style Pro pedometer at baseline and were encouraged to progressively increase steps and ultimately to achieve 10,000 steps per day. Consultations were regularly held to discuss daily walking targets, and were encouraged to exercise and to integrate physical activity into activities of daily living.
Both interventions provided health promotion counseling [Citation20] including clinical advice concerning symptom management and feedback regarding physiological outcomes.
Outcomes
BCRL was objectively assessed (inter-arm volume % difference and extracellular fluid) at baseline, 12 and 39 weeks, by medical technicians at the Department of Clinical Physiology and Nuclear Medicine, Rigshospitalet. Muscle strength outcome measures and self-reported outcomes were obtained by research assistants at the University Hospitals Center for Health Research, Rigshospitalet at baseline, 6, 12 and 39 weeks.
Inter-arm volume % difference
Arm volume was obtained using Dual energy X-ray absorptiometry (DXA) (Lunar Prodigy Advanced Scanner, GE Healthcare, Madison, WI). DXA provides a sensitive measure of tissue composition using a three-compartment model [Citation25,Citation26]. Lying supine on the scan-table with arms slightly abducted and hands in a mid-prone position, total body scans were performed. From the total body scans, estimated arm volumes were calculated using previously derived densities (fat - 0.9 g/ml, lean mass −1.1 g/ml, bone mineral content - 1.85 g/ml)) with the region of interest extending from the gleno-humeral joint to the finger tips [Citation25,Citation26]. Inter-arm volume % differences (at-risk arm minus unaffected arm/unaffected arm * 100) were then calculated. To ensure accuracy, participants were scanned fasting at the same time of day (mornings) at all assessments.
L-Dex
To ensure obtainment of high-quality objective measures of extracellular fluid, bioimpedance spectroscopy (BIS) (SFB7, Impedimed, Brisbane, Australia) [Citation27] was added to the test battery after commencement of the study, with data consecutively collected from participant 71 forward. Measurements were performed immediately after DXA scans as previously described [Citation28,Citation29]. The ratio of impedance (at R0) between the at-risk and non-affected arm was calculated and converted into an L-Dex score taking arm dominance into account [Citation30].
Self-reported swelling
Participants were asked if they had observed a difference in size between their surgical- and nonsurgical side within the last week (dichotomous scale – yes/no). If they answered yes, they were then asked to report where (fingers, hand, forearm, upper arm, breast, torso). For the purpose of binary analysis, categories were divided into ‘extremity’ (fingers, hand, forearm, upper arm) and ‘body’ (breast, torso).
Self-reported BCRL symptoms
The severity of lymphedema symptoms on the surgical side was monitored using a numeric rating scale (NRS) [Citation31]. Participants rated perceptions of swelling, heaviness, pain and tightness within the last week on a scale from 0 (no discomfort) to 10 (very severe discomfort) [Citation32].
Muscular strength
Maximal upper-extremity strength was assessed using the 1 RM strength test [Citation33] in the chest press exercise. Prior to the 1 RM attempt, a warm-up consisting of 8–10 repetitions using a low weight ensuring no muscle fatigue was performed. Hereafter, load was increased based on ease of performance, with one repetition lifted of each load, until the participant was unable to lift a respective load.
Breast cancer-specific quality of life (QOL)
The 23-item European Organization for Research and Treatment of Cancer (EORTC QLQ) breast cancer module (BR23) [Citation34], version 3.0, was used to assess breast cancer-specific QOL. This validated breast cancer-specific module includes four functional subscales, and four symptom specific subscales. The raw scores were summed and converted to a score out of 100 [Citation34,Citation35].
Blinding
All data collection and subsequent data entry were performed blinded to group allocation by study assessors. Further, all data analyses were performed with no knowledge of group allocation by a statistician with no other affiliation to the study.
Statistical analysis
Descriptive statistics for continuous variables included means ± standard deviations (SD) for normally distributed data or median with interquartile range (IQR) for non-normally distributed data. Categorical variables as well as BCRL point prevalence (defined as L-Dex >10, inter-arm volume difference >5%, self-reported observation of swelling) are presented as counts (percentages). Intention-to-treat analyses were performed using linear mixed models with a heterogeneous autoregressive (1) covariance structure to estimate changes over time in each group. An exchangeable correlation structure was used to model the within-subject correlation of repeated measurements over time and across interventions, incorporating all available data including participants with incomplete data. Also, effect sizes were calculated for muscular strength [Citation36]. A two-sided significance level was set at 0.05 for outcomes where superiority was hypothesized.
A priori, equivalence margins were chosen for BCRL outcomes. If the mean difference and interval between the upper- and lower-confidence limits was within the predetermined equivalence margin, equivalence between interventions was declared [Citation37]. An equivalence margin of ±3.0% was used for inter-arm volume % differences based on findings from Stout et al., [Citation38] that volume increases of >3% from pre-operative measures were indicative of sub-clinical BCRL. For L-Dex, the margin of equivalence was set at ±5.0 units based on normative data indicating that L-Dex scores fluctuate between 9–11 units [Citation30]. For lymphedema symptom severity an equivalence margin was set at ±1.0 points based on data that suggest a 2 point or 30% change to be clinically meaningful for pain [Citation31]. The principle of confidence interval inclusion [Citation39] was used to calculate two one-sided upper- and lower-95% confidence limits for L-Dex, inter-arm volume % differences, and BCRL symptom outcomes (reported as 90% confidence limits). A per-protocol analysis was performed to determine equivalence of BCRL outcomes of participants with an adherence rate >65% to the HIGH intervention (n = 33). Means and 90% CI were calculated and compared with the predetermined equivalence margins. Analyses were conducted using Statistical Analysis Software (SAS) version 9.4.
Results
Between January 2014 and July 2016, 153 of 391 (39%) eligible women receiving adjuvant chemotherapy for breast cancer were recruited (). Baseline characteristics were balanced between the two intervention groups ().
Table 1. Baseline characteristics of 153 women receiving adjuvant chemotherapy for primary breast cancer. Copenhagen University Hospitals, Herlev and Rigshospitalet, 2014–2016.
For participants with and without L-Dex data, baseline characteristics were balanced with the exception of chemotherapy regime, as more participants with L-Dex data had received paclitaxel (13 (20%) vs. 6 (7%), respectively). Further, differences were seen in the mean BMI of participants with and without inter-arm volume data as the body dimensions of some participants exceeded the DXA scan area (24 ± 4 kg/m2 with DXA, vs. 32 kg/m2 ± 5 without DXA).
Retention, adherence and adverse events
Outcome data were available for 130 participants (85%) at 12-weeks postintervention, and for 121 (79%) at the 39 week follow-up (). On average, participants in the HIGH group attended 66% (±18) of the planned exercise sessions. Four women never partook in the intervention and an additional six withdrew shortly after initiation of the program. Adherence to resistance exercise prescription of the upper-extremity corresponded to a median load of 10 RM during the first two weeks. From week three forward (heavy-load period), loads corresponded to 7 RM. Comparatively loads lifted for the leg press were 14 RM and 8 RM, respectively.
Eight participants experienced minor adverse events related to exercise (see Møller et al., in submission), while no adverse events prompting medical attention were reported during the study period. In all, 11 participants (HIGH n = 6 (8%) vs. LOW n = 5 (6%)) developed swelling during the 12 week intervention and were referred to lymphedema therapists. At 39 weeks, seven of these participants had received treatment for BCRL following the intervention, while three had not, and one was lost-to follow-up. During the intervention period, resistance exercise prescription was modified for one participant in the HIGH group (reduced loads to 10–15 RM), whereas the other five continued lifting loads corresponding to 5–8 RM.
Lymphedema
Point prevalence: While point prevalence of BCRL varied depending on the method of assessment (), it was similar between the HIGH and LOW group at all time points, for any given method of assessment.
Table 2. Point prevalence (N (%)) of lymphedema (L-Dex >10, inter-arm volume difference > 5% and self-reported swelling) based on all available data for each outcome, in 153 women receiving adjuvant chemotherapy for primary breast cancer. Copenhagen University Hospitals, Herlev and Rigshospitalet, 2014–2016.
Self-reported diagnosis of BCRL at baseline: Five (3%) participants (LOW (n = 4) vs. HIGH (n = 1)) reported at baseline that they had been diagnosed with-, and were receiving treatment for BCRL.
The one participant in the HIGH group continued treatment by a lymphedema therapist throughout the 12-week intervention and undertook the resistance exercise protocol without need for modification (e.g., less load). At 12 weeks, she reported no observations of swelling along with reductions in symptoms. No DXA or BIS data were available for this participant.
Inter-arm volume % difference: Nonequivalence was observed at all time points for inter-arm volume % differences with deviations exceeding equivalence margins favoring the HIGH group (). These observations were consistent with findings from the per-protocol analysis (Supplementary Table 3).
Table 3. Equivalence between groups for lymphedema outcomes in 153 women receiving adjuvant chemotherapy for primary breast cancer. Copenhagen University Hospitals, Herlev and Rigshospitalet, 2014–2016.
L-Dex: Equivalence between groups was found at both 12 and 39 weeks (). Equivalence to the predetermined equivalence margin in the per-protocol analysis at 12 weeks was also observed (Supplementary Table 3), while the upper CI exceeded the margin of equivalence at 39 weeks, rendering nonequivalence.
BCRL symptoms: Equivalence between groups was found in all symptoms except for pain post-intervention, and tightness and pain at the 39-week follow-up (all favoring reductions for those in the HIGH group) (). Results of the per-protocol analysis also indicated equivalence for heaviness and swelling at 12 and 39 weeks, as well as pain postintervention (Supplementary Table 3). Nonequivalence was found for pain at 39 weeks and tightness at both 12 and 39 weeks.
Data detailing absolute values for BCRL outcomes, at all time points for completers and noncompleters, can be found in Supplementary Table 2.
Muscular strength
A significant change in maximal upper-extremity strength was observed for participants in the HIGH group at all follow-up assessments (). At 6- and 12-week follow-up, these strength increases were significantly greater compared to those in the LOW group and corresponded to effect sizes of 0.55 (95% CI 0.40–0.75), 0.55 (0.35–0.70) and 0.35 (0.15–0.55) at 6, 12 and 39 weeks follow-up, respectively.
Table 4. Within group changes and between group differences for upper-extremity strength and breast cancer-specific functional and symptom domains in 153 women receiving adjuvant chemotherapy for primary breast cancer. Copenhagen University Hospitals, Herlev and Rigshospitalet, 2014–2016.
Breast cancer-specific QOL
No between group differences were observed for any subscale score of the EORTC QLQ-BR23 at all assessments ( and Supplementary Table 4). Nonetheless, both groups reported declines in breast symptoms at 6 and 12 weeks and arm symptoms at 6 weeks follow-up, while reductions in arm symptom at 12 weeks only was seen in the HIGH group.
Discussion
In line with the hypothesis, we found similar point prevalent cases of BCRL between groups, as well as similar between group L-Dex scores and perceptions of heaviness, swelling and tightness post-intervention. While equivalence was not demonstrated in inter-arm volume % differences or self-reported pain, the negative deviations indicated reductions of these outcomes, favoring the HIGH group. Notably, per-protocol analysis of HIGH participants with >65% adherence consistently supported equivalence to- or reductions beyond the predetermined equivalence margins, adding strength to the findings. Additionally, clinically relevant within group reductions in breast and arm symptoms were found in the HIGH group [Citation40] at both 6 and 12 weeks.
This work extends the results of previous research finding a similar acute lymphatic response to one bout of low-and heavy-load resistance exercise [Citation41], to repeated exposure of resistance exercise with heavy loads. Further, our results are in agreement with findings of Cormie et al. [Citation32,Citation42] who demonstrated the safety of heavy-load (75–85% of 1 RM using 6–10 RM) resistance exercise in breast cancer survivors with stable BCRL. As such, these results add to a growing and consistent evidence base which suggests that resistance exercise is safe for those with or at-risk of developing lymphedema [Citation14,Citation16–18].
Participation in HIGH significantly improved upper-extremity strength with increases corresponding to 17%, 13%, and 7% at 6, 12, and 39 weeks, respectively, compared to 3% at all assessments in the LOW group. These are relevant findings as upper-extremity strength in breast cancer survivors (without intervention) has been found to be 12–16% lower compared to healthy women [Citation43]. As such, the present study indicates that participation in heavy-load resistance exercise during chemotherapy can mitigate declines in muscle strength.
At 39 weeks follow-up, we found that the longer-term effect of the LOW and HIGH intervention was similar between groups or indicated reductions favoring the HIGH group for all outcomes. These findings were consistent with the per-protocol analysis of the HIGH group, with the exception of L-Dex and pain as upper CI’s indicated a slight increase beyond the predetermined equivalence margin. However, in general, care should be taken when interpreting the 39-week follow-up results as no data regarding upper-extremity resistance exercise behavior was collected post-intervention, which is a limitation. Consequently, we cannot determine whether effects seen at 39 weeks were related to resistance exercise or other unknown factors.
Additional limitations should be considered when interpreting findings. First, usual care in Denmark includes municipality lead rehabilitation programs and was therefore available for all participants. These programs are generally offered one to two times per week and include resistance exercise at low to moderate intensities. It is thus likely that a proportion of those in the LOW group also participated in resistance-based exercise at low to moderate loads during the intervention period. While there exists uncertainty as to the extent of this potential bias, the impact on findings would likely dilute differences between interventions. In addition, though groups were statistically balanced at baseline, it should be noted that more participants in the LOW group had ALND and had been diagnosed with BCRL at baseline potentially swaying the results in favor of the HIGH group. Further, in all 28% of the sample are missing inter-arm volume data due to body dimensions exceeding the DXA scan table, why caution in generalizing these findings to obese women is required. Future studies should be aware of this limitation and perform separate arm scans [Citation44] as an alternative for these individuals. Also, extracellular fluid measured by BIS was added to the test battery from participant 71 forward (54% of the sample). Therefore, complete data were not available for these two outcomes. Nonetheless, the comprehensive assessment of BCRL used in this study (i.e., two objective assessment methods, alongside self-report) ensured that each participant contributed 100% data for at least one outcome measure.
Strengths of the study include the randomized design as well as blinded assessments and analyses. Further, intention-to-treat analyses were performed for all outcomes as well as per-protocol analyses of HIGH participants with adherence rates >65% for BCRL outcomes, and consistency in findings was observed. In addition, this study targeted breast cancer survivors who were physically inactive before diagnosis. This is important as fear of lymphedema has been identified as a barrier for physical activity and especially vigorous or strength activities [Citation45], which in turn may lead to avoidance and non-adoption of regular physical activity, further increasing risk of BCRL for this significant group [Citation1].
In conclusion, novel findings from this study suggest that breast cancer survivors who are physically inactive before diagnosis benefit from and can safely participate in a multimodal intervention that includes supervised, heavy-load resistance exercise of the upper-extremities. Notably, the findings are particularly relevant for the majority of breast cancer survivors who receive taxane-based chemotherapy, with generalized edema and ensuing arm swelling as a known side-effect of this cytostatic agent. As such, these findings can be used to encourage adoption of exercise including heavy-load resistance exercise during breast cancer treatment.
Supplemental Material
Download (66.1 KB)Acknowledgments
We greatly thank the staff at the Department of Clinical Physiology and Nuclear Medicine at the Copenhagen University Hospital, Rigshospitalet for collaboration in data collection, and the participants for their dedication to the study.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515.
- Boyages J, Kalfa S, Xu Y, et al. Worse and worse off: the impact of lymphedema on work and career after breast cancer. Springerplus. 2016;5:657.
- Morgan PA, Franks PJ, Moffatt CJ. Health-related quality of life with lymphoedema: a review of the literature. Int Wound J. 2005;2:47–62.
- Fu MR, Ridner SH, Hu SH, et al. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. 2013;22:1466–1484.
- Schmidt ME, Wiskemann J, Ulrich CM, et al. Self-reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow-up of two randomized exercise intervention trials. Acta Oncol. 2017;56:618–627.
- Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118:2277–2287.
- Vagenas D, DiSipio T, Battistutta D, et al. Weight and weight change following breast cancer: evidence from a prospective, population-based, breast cancer cohort study. BMC Cancer. 2015;15:28.
- Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2006;9:CD005001.
- Makari-Judson G, Judson CH, Mertens WC. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J. 2007;13:258–265.
- Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700.
- Christensen JF, Jones LW, Andersen JL, et al. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25:947–958.
- Csapo R, Alegre LM. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: a meta-analysis. Scand J Med Sci Sports. 2016;26:995–1006.
- American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708.
- Paramanandam VS, Roberts D. Weight training is not harmful for women with breast cancer-related lymphoedema: a systematic review. J Physiother. 2014;60:136–143.
- Schmitz KH. Balancing lymphedema risk: exercise versus deconditioning for breast cancer survivors. Exerc Sport Sci Rev. 2010;38:17–24.
- Cheema BS, Kilbreath SL, Fahey PP, et al. Safety and efficacy of progressive resistance training in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;148:249–268.
- Nelson NL. Breast cancer-related lymphedema and resistance exercise: a systematic review. J Strength Cond Res. 2016;30:2656–2665.
- Keilani M, Hasenoehrl T, Neubauer M, et al. Resistance exercise and secondary lymphedema in breast cancer survivors-a systematic review. Support Care Cancer. 2016;24:1907–1916.
- Ammitzboll G, Johansen C, Lanng C, et al. Progressive resistance training to prevent arm lymphedema in the first year after breast cancer surgery: results of a randomized controlled trial. Cancer 2019;125:1683–1692.
- Moller T, Lillelund C, Andersen C, et al. At cancer diagnosis: a 'window of opportunity' for behavioural change towards physical activity. A randomised feasibility study in patients with colon and breast cancer. BMJ Open. 2013;3:e003556–24189081.
- Moller T, Lillelund C, Andersen C, et al. The challenge of preserving cardiorespiratory fitness in physically inactive patients with colon or breast cancer during adjuvant chemotherapy: a randomised feasibility study. BMJ Open Sport Exerc Med. 2015;1:e000021–27900123.
- Danish Health and Medical Authority. Physical activity: recommendations for adults (18-64 years old) 2013. Available from: http://www.sst.dk/English/Health_promotion/Physical_activity/Recommendations_for_adults.aspx.
- Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ 2009;339:b3410–19826172.
- Bloomquist K, Karlsmark T, Christensen KB, et al. Heavy resistance training and lymphedema: prevalence of breast cancer-related lymphedema in participants of an exercise intervention utilizing heavy load resistance training. Acta Oncol. 2014;53:216–225.
- Newman AL, Rosenthall L, Towers A, et al. Determining the precision of dual energy x-ray absorptiometry and bioelectric impedance spectroscopy in the assessment of breast cancer-related lymphedema. Lymphat Res Biol. 2013;11:104–109.
- Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7:3–10.
- Ward LC, Dylke E, Czerniec S, et al. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol. 2011;9:47–51.
- Cornish BH, Jacobs A, Thomas BJ, et al. Optimizing electrode sites for segmental bioimpedance measurements. Physiol Meas. 1999;20:241–250.
- Bloomquist K, Hayes S, Adamsen L, et al. A randomized cross-over trial to detect differences in arm volume after low- and heavy-load resistance exercise among patients receiving adjuvant chemotherapy for breast cancer at risk for arm lymphedema: study protocol. BMC Cancer. 2016;16:22–517.
- Hayes S, Janda M, Steele M, et al. Identifying diagnostic criteria for upper- and lower-limb lymphoedema Impedimed Limited: Queensland University of Technology Faculty of Health, School of Public Health and Social Work and Institute of Health and Biomedical Innovation; 2016 [updated 3 July 2017; cited 2017 31 May]. 17]. Available from: https://eprints.qut.edu.au/view/person/Hayes,_Sandra.html#group_report.
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63:S240–S52.
- Cormie P, Galvao DA, Spry N, et al. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivors. Integr Cancer Ther. 2013;12:423–432.
- Levinger I, Goodman C, Hare DL, et al. The reliability of the 1RM strength test for untrained middle-aged individuals. J Sci Med Sport. 2009;12:310–316.
- Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. Jco. 1996;14:2756–2768.
- Nguyen J, Popovic M, Chow E, et al. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review. J Comp Eff Res. 2015;4:157–166.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. USA: Lawrence Erlbaum Associates;1988.
- Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26:192–196.
- Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819.
- Westlake WJ. Use of confidence intervals in analysis of comparative bioavailability trials. J Pharm Sci. 1972;61:1340–1341.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144.
- Bloomquist K, Oturai P, Steele ML, et al. Heavy-load lifting: acute response in breast cancer survivors at risk for lymphedema. Med Sci Sports Exerc. 2018;50:187–195.
- Cormie P, Pumpa K, Galvao DA, et al. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surviv. 2013;7:413–424.
- Klassen O, Schmidt ME, Ulrich CM, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle. 2017;8:305–316.
- Gjorup C, Zerahn B, Hendel HW. Assessment of volume measurement of breast cancer-related lymphedema by three methods: circumference measurement, water displacement, and dual energy X-ray absorptiometry. Lymphat Res Biol. 2010;8:111–119.
- Sander AP, Wilson J, Izzo N, et al. Factors that affect decisions about physical activity and exercise in survivors of breast cancer: a qualitative study. Phys Ther. 2012;92:525–536.